Andrographolide polymer micelle, preparation method and medicinal application thereof
A technology of andrographolide and polymer glue is applied in the directions of pharmaceutical formulations, antitumor drugs, drug combinations, etc., to achieve the effects of masking severe bitterness, stable product quality, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 amphiphilic block copolymer
[0037] (1) Methoxy polyethylene glycol-polylactide block copolymer (mPEG 3400 -PLA 1800 ): Add 50g mPEG (number average molecular weight 3400) into a polymerization bottle, heat to 100°C, vacuum dehydrate for 2 hours, add 36mg stannous octoate and 36g D,L-lactide (D,L-LA), vacuum seal Reaction vial, react at 125°C for 15h. Dissolve the reactants with dichloromethane, add a large amount of ether to fully precipitate the polymer, filter and dry in vacuum.
[0038] (2) Benzoyl-terminated methoxypolyethylene glycol-polylactide block copolymer (mPEG 3400 -PLA 1800 -Bz): take 38g mPEG 3400 -PLA 1800 Add 190ml of ethyl acetate to dissolve, add 2.8ml of triethylamine, add 2.3ml of benzoyl chloride dropwise under stirring, heat the above solution to boiling after the dropwise addition, continue the reflux reaction for 6h, filter to remove insoluble matter, add a large amount of ether to precipitate The polymer ...
Embodiment 2
[0043] Embodiment 2 andrographolide polymer micelles lyophilized preparation preparation
[0044] (1) mPEG 5000 -PCL 4000 -Preparation of Ac / andrographolide polymer micelles and its lyophilized preparation
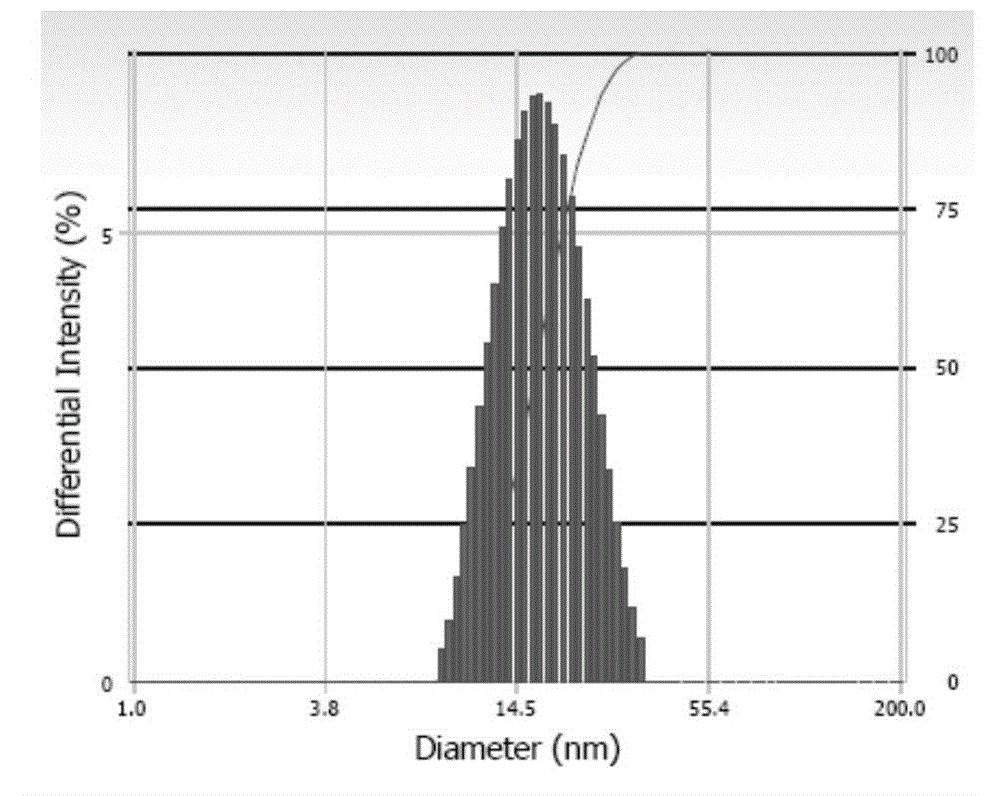
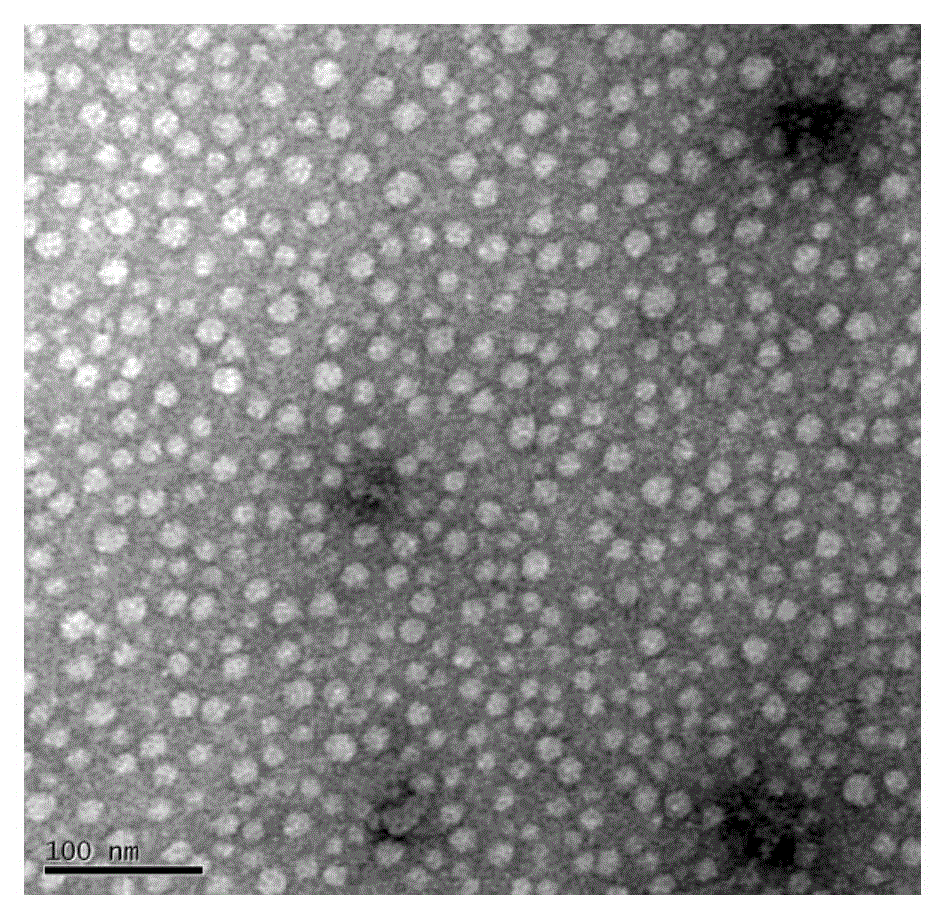
[0045] Get the mPEG prepared in 150mg embodiment 1 (4) 5000 -PCL 4000 -Ac and 30mg of andrographolide were dissolved in 2ml of tetrahydrofuran, and 5ml of ultrapure water was slowly added dropwise under stirring. After the dropwise addition, the solution was stirred at room temperature overnight to remove the organic solvent to obtain clear paclitaxel micelles with obvious blue opalescence 120 mg of mannitol was added to the solution, and the resulting solution was filtered through a 0.22 μm sterile membrane and freeze-dried to obtain a freeze-dried powder of andrographolide polymer micelles. According to HPLC analysis, the drug encapsulation rate is 96.5%, the drug loading capacity is greater than 15.4%, the average particle size is 18.7nm, and the dispersion coeffici...
Embodiment 3
[0050] The pharmacokinetics of embodiment 3 andrographolide polymer micelles in rat body
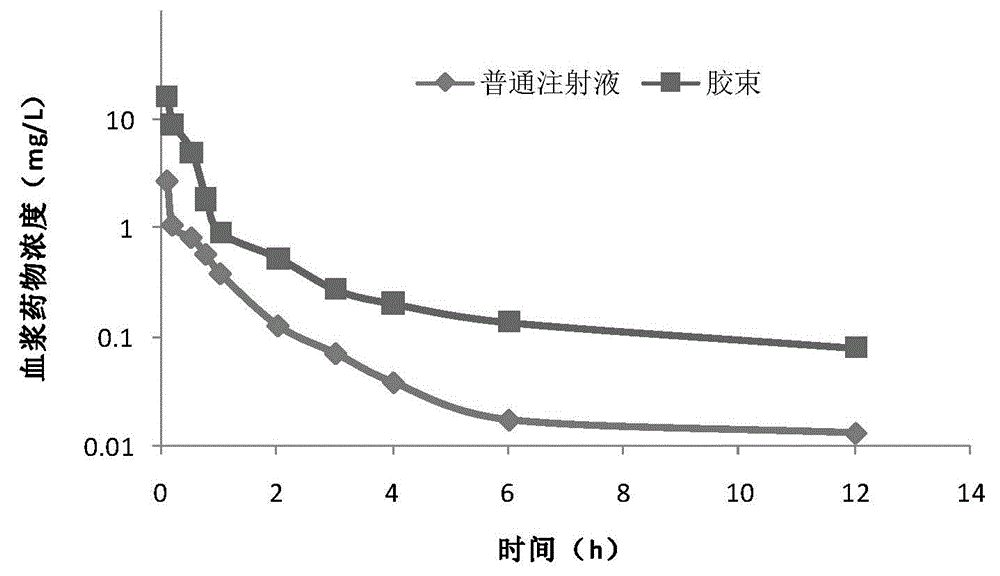
[0051] (1) Plasma drug-time curve of andrographolide polymer micelles rat tail vein injection
[0052] Test drug: andrographolide polymer micelle lyophilized preparation was prepared according to Example 2 (1), and was dissolved with physiological saline before use to make a solution containing 10 mg of andrographolide per 1 ml. In addition, DMSO was used as a solvent to dissolve andrographolide to make an andrographolide injection containing 10 mg of andrographolide per 1 ml as a reference preparation.
[0053] Dosing and blood collection scheme: 12 SD rats, weighing 200-220g, were randomly divided into two groups, with 6 rats in each group, half male and half male, and were injected with andrographolide polymer micelle solution and DMSO solution through tail vein respectively. , the dosage is 20mg / kg in terms of andrographolide. At different time points after the administration, 0.5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com