Synthesis method of cefpirome sulfate
A technology of cefpirome sulfate and synthesis method, applied in directions such as organic chemistry, can solve the problems of few reaction steps and high production cost, and achieve the effects of few reaction steps, convenient operation and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
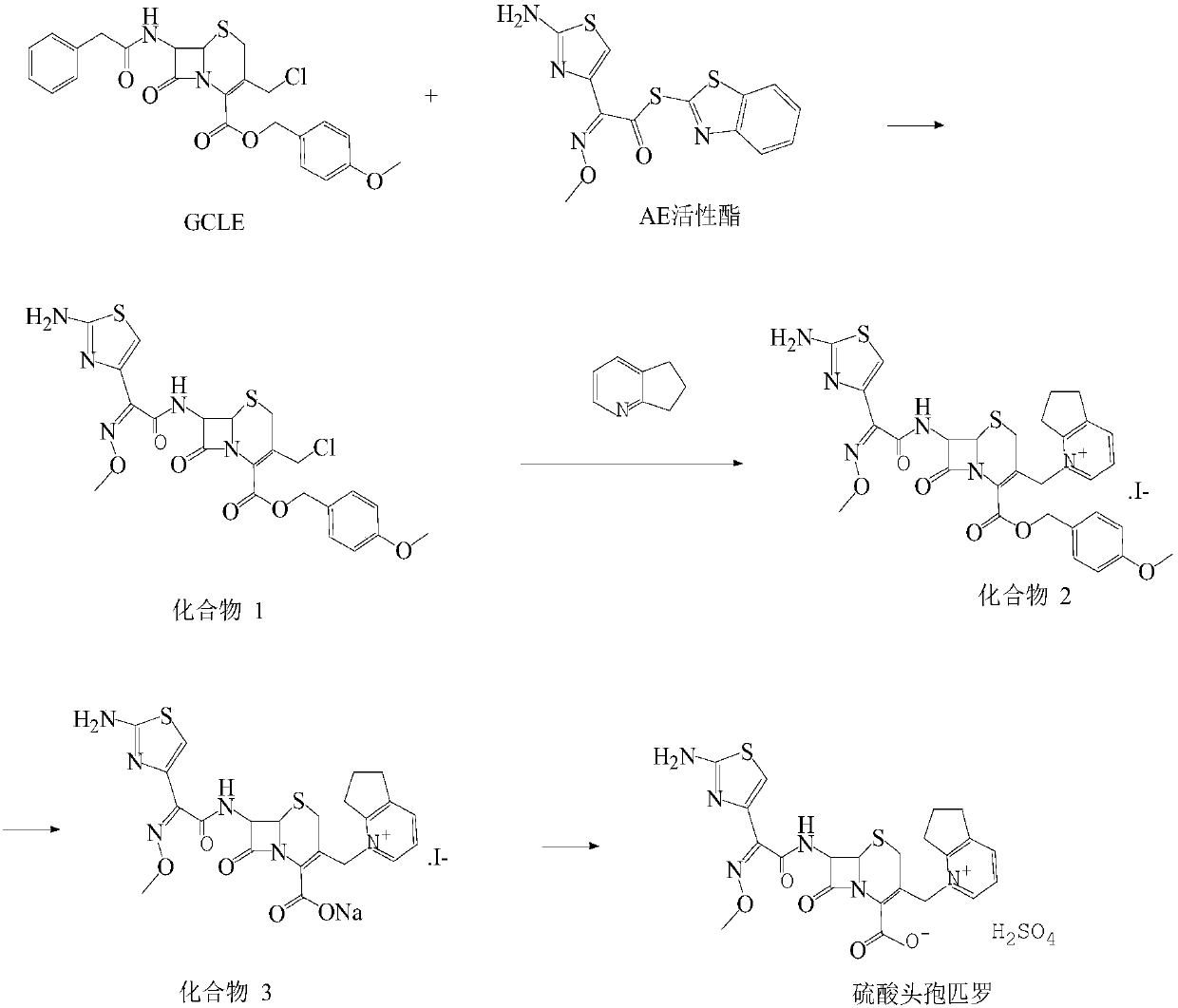
[0036] (1) Synthesis of Compound A
[0037] 25g of GCLE and 17.9g of AE active ester were added to a mixed solvent of 400ml of dichloromethane and 80ml of ethanol, stirred and cooled to -5°C, and 7.7ml of triethylamine and 1.2ml of pyridine were added dropwise. At this temperature, stir until dissolved, continue to react for 2h, heat up to 18°C, add 300ml of purified water, stir for 15min, let stand for 10min, separate the liquid, collect the aqueous phase, add 180ml of purified water to the organic phase, stir for 15min, let stand for 10min, Separation, combining the two aqueous phases, adding 20% dilute hydrochloric acid dropwise to adjust the pH to 3.0 for crystallization, cooling to 5°C, filtering, rinsing with ethanol, and vacuum drying to obtain 23.5 g of compound A with a yield of 82.95%.
[0038] (2) Synthesis of Compound B
[0039] Add 7.7 g of sodium iodide as an activator to 380 ml of acetone and stir until it dissolves clearly, add compound A, stir at room tempe...
Embodiment 2
[0043] (1) Synthesis of Compound A
[0044] 25g of GCLE and 17.9g of AE active ester were added to a mixed solvent of 400ml of dichloromethane and 80ml of ethanol, stirred and cooled to -5°C, and 7.7ml of triethylamine and 1.2ml of pyridine were added dropwise. At this temperature, stir until dissolved, continue the reaction for 2.5h, heat up to 18°C, add 300ml of purified water, stir for 15min, let stand for 10min, separate the liquid, collect the aqueous phase, add 180ml of purified water to the organic phase, stir for 15min, and let stand for 10min , separate the liquids, combine the two aqueous phases, add 15% dilute hydrochloric acid dropwise to adjust the pH to 3.0 for crystallization, cool to 5°C, filter, rinse with ethanol, and vacuum dry to obtain 22.5 g of compound A with a yield of 81.42%.
[0045] (2) Synthesis of Compound B
[0046] Add 7.7 g of sodium iodide as an activator to 380 ml of acetone and stir until it dissolves clearly, add compound A, stir at room te...
Embodiment 3
[0050](1) Synthesis of Compound A
[0051] 25g of GCLE and 17.9g of AE active ester were added to a mixed solvent of 400ml of dichloromethane and 80ml of ethanol, stirred and cooled to -3°C, 2.1g of sodium bicarbonate was added, 1.2ml of pyridine was added dropwise, and the temperature when the dropwise addition was completed did not exceed 0°C, Control the temperature, stir until dissolved, continue the reaction for 3h, heat up to 19°C, add 300ml of purified water, stir for 15min, stand for 10min, separate the liquid, collect the aqueous phase, add 180ml of purified water to the organic phase, stir for 15min, and let stand for 10min , the liquids were separated, the two aqueous phases were combined, 22% dilute hydrochloric acid was added dropwise to adjust the pH to 3.0 for crystallization, cooled to 5°C, filtered, rinsed with ethanol, and dried in vacuo to obtain 24.2 g of compound A with a yield of 83.23%.
[0052] (2) Synthesis of Compound B
[0053] Add 8.9 g of activato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com