Method for synthesizing cefpirome sulfate
A technology of cefpirome sulfate and a synthetic method, which is applied in the field of medicine, can solve problems such as difficult product purification, low yield, and excessive side reactions of the method, and achieve the effects of stable product quality, reduced degradation and damage, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
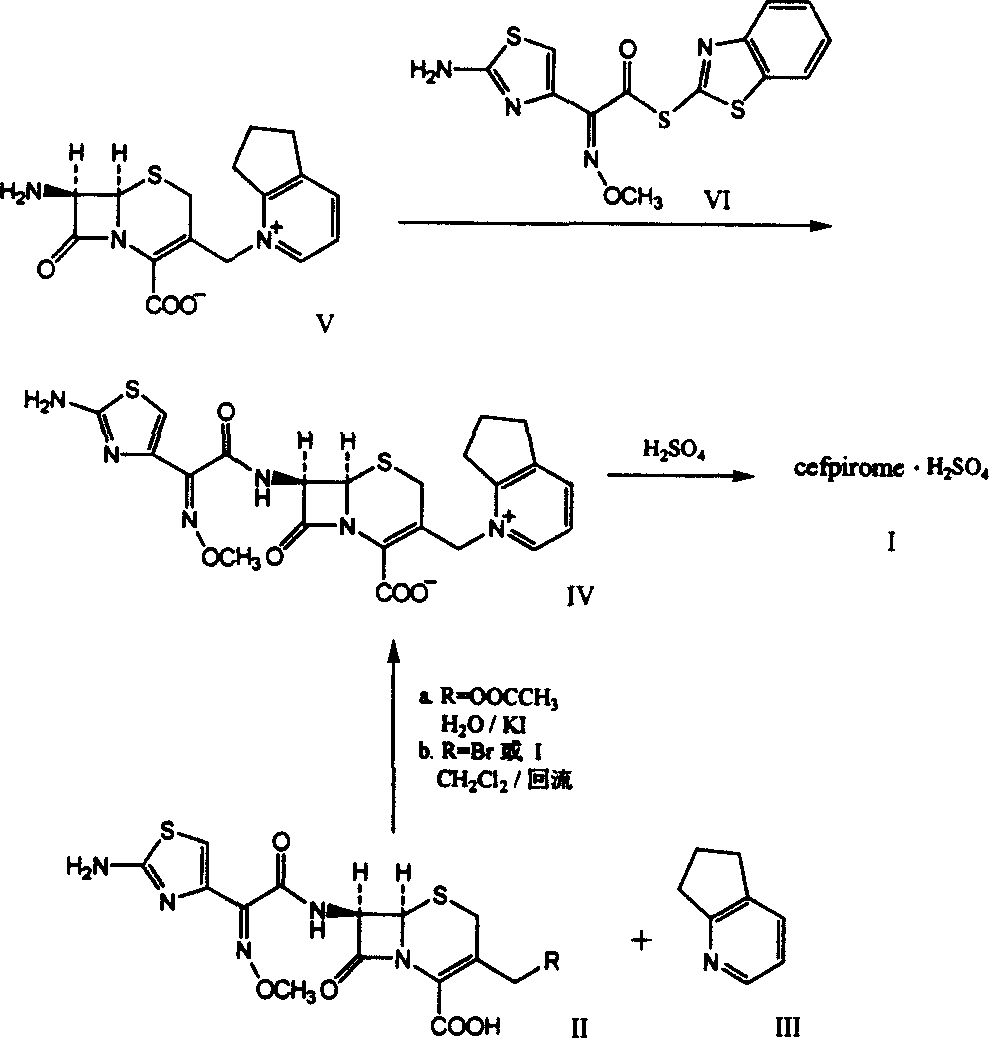
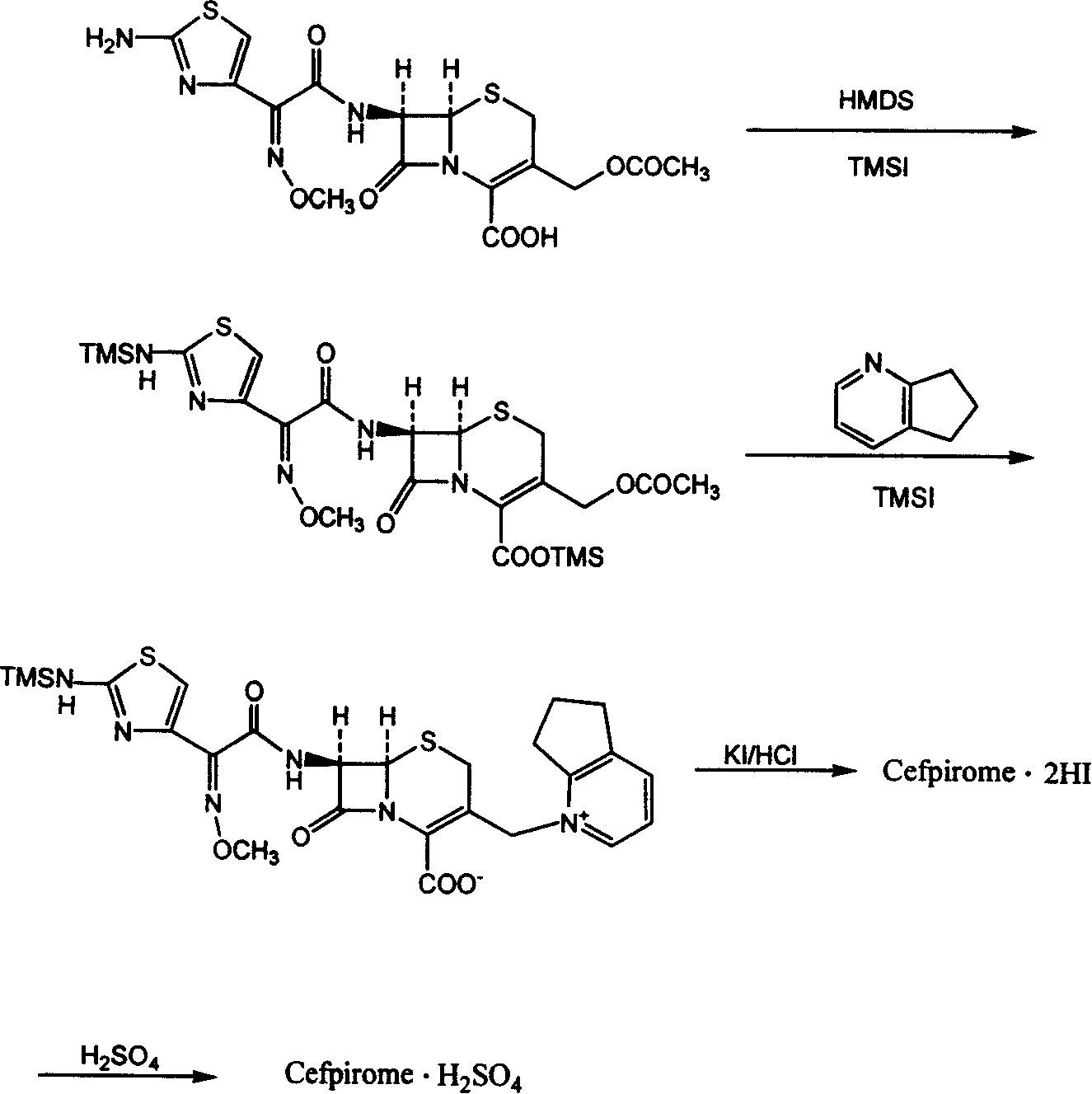
[0016] Step 1. 7-[2-(2-Aminothioxime-4-yl)-2-(cis)-methoxyiminoacetamido]-3-[(2,3-cyclopenta-1-pyridine ) methyl] - the preparation of cef-3-ene-4-carboxylic acid dihydroiodide (cefpirome dihydroiodide):
[0017] In a 10L reaction flask, add 1.1 moles of cefotaxime acid, 36 moles of dichloromethane, 2.0 moles of hexamethyldisilazane, and 0.035 moles of iodotrimethylsilane, and reflux for 7 hours under nitrogen protection. After cooling the solution, add it to the pre-prepared mixture of 40 moles of dichloromethane, 4.3 moles of iodotrimethylsilane, and 3.8 moles of 2,3-cyclopentenopyridine under the condition of an ice-water bath and nitrogen protection, and continue After reflux and stirring for 2 hours, under cooling and stirring in an ice-water bath, add a solution obtained by dissolving 4 moles of potassium iodide in 1.68 L of 3N HCl within 5 minutes, stir at the same temperature for 2 hours, place at 4°C overnight, and collect the formed precipitate by filtration , the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com