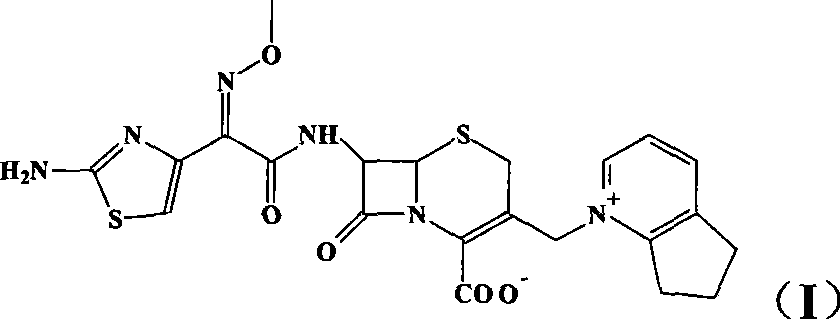
Method for preparing high-purity cefpirome sulfate by sodium salt precipitation method
A technology of cefpirome sulfate and precipitation method, applied in organic chemistry, antibacterial drugs, etc., can solve the problems of high energy consumption and achieve the effects of large equipment output, good industrial application value, and short production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 300g of cefpirome sulfate crude product (0.49mol, the weight is based on anhydrous matter) to 1500mL of water at room temperature (15-25°C), add about 20% sodium hydroxide aqueous solution dropwise under vigorous stirring until the pH of the system is 6.5- Between 7.0 (the amount of sodium hydroxide is about 39g, 0.98mol). Then add 3000mL acetone and an appropriate amount of activated carbon, and stir for 30 minutes. The solid was isolated by centrifugation or suction filtration. Wash the filter cake with 800 mL of acetone-water 2:1 (v / v) mixed solution, and combine the filtrates. Add 30% H to the filtrate with stirring 2 SO 4 to pH1.0-1.5. Then 9 L of acetone was added. After the crystals were precipitated, the temperature of the system was lowered to 5-10°C, and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40° C. to obtain about 270 g of white crystalline powder. The purity is above 99%, ...
Embodiment 2
[0031] At room temperature (15-25°C), add 300g of cefpirome sulfate crude product (0.49mol, weight based on anhydrous matter) into 1500mL of water, and then add 150ml of acetone. Add NaHCO in batches with stirring 3 82.3 g (0.98 mol). Stir for 20 minutes. Then add 2850mL acetone and appropriate amount of activated carbon, and stir for 30 minutes. The solid was isolated by centrifugation or suction filtration. Wash the filter cake with 600 mL of acetone-water 2:1 (v / v) mixed solution, and combine the filtrates. Add 50% H to the filtrate with stirring 2 SO 4 to pH1.0-1.5. Then 9 L of acetone was added. After the crystals were precipitated, the temperature of the system was lowered to 5-10°C, and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40° C. to obtain about 270 g of white crystalline powder. Result is with embodiment 1.
Embodiment 3
[0033] Add 300g of cefpirome sulfate crude product (0.49mol, weight based on anhydrous matter) to 1500mL of water at room temperature (15-25°C), and then add 100ml of acetone. Add Na in batches under stirring 2 CO 3 48 g (0.45 mol). Stir for 20 minutes. Then add 3000mL acetone and an appropriate amount of activated carbon, and stir for 30 minutes. The solid was isolated by centrifugation or suction filtration. Wash the filter cake with 600 mL of acetone-water 2:1 (v / v) mixed solution, and combine the filtrates. Add 40% H to the filtrate with stirring 2 SO 4 to pH1.0-1.5. Then 9 L of acetone was added. After the crystals were precipitated, the temperature of the system was lowered to 5-10°C, and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40° C. to obtain about 270 g of white crystalline powder. Result is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com