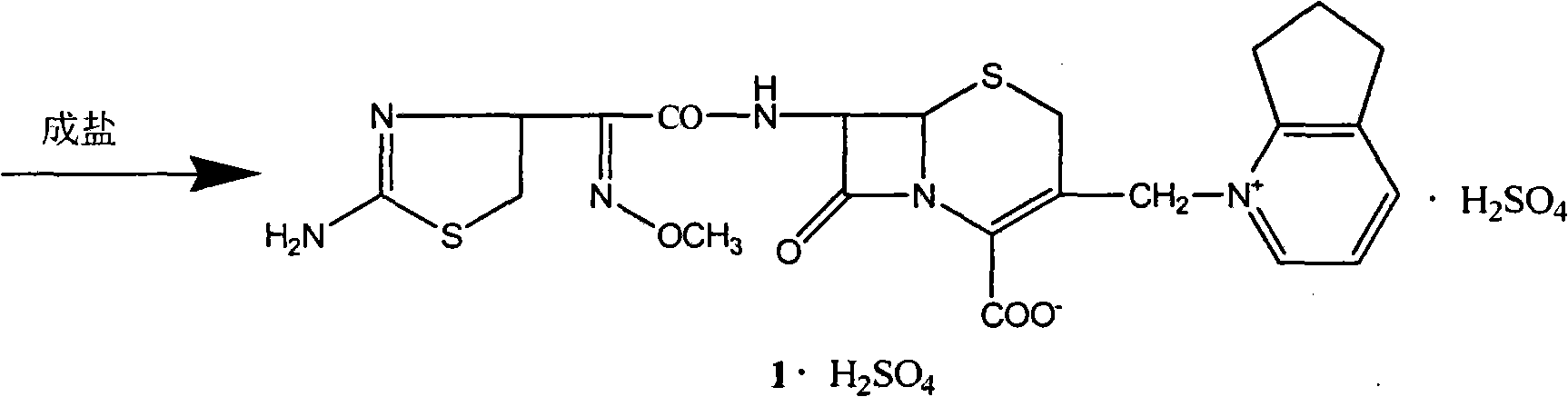
Method for synthesizing antibiotic cefpirome sulfate
A technology of cefpirome sulfate and synthetic method, applied in the direction of antibacterial drugs, organic chemistry, etc., can solve the problems of harsh reaction conditions, high cost of raw materials, long reaction time, etc., and achieve simple process conditions, stable product quality, and product yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Synthesis of Example 1 7-ACP
[0014] In the flask, add 40g (0.14mol) 7-aminocephalosporanic acid (7-ACA), 27.4g (0.17mol) hexamethyldisilazane amine, N,N-dimethylformamide (DMF) 1ml, three Methyl iodosilane 1ml, dichloromethane 300mL, stirred at 20-25°C for 6-10 hours. Then the temperature was lowered to 0° C., 27 mL of tetrahydrofuran was added, and 63 g (0.3 mol) of iodotrimethylsilane was added dropwise, and then, the reaction was stirred for 4-5 hours.
[0015] Then, 30 g (0.24 mol) of 2,3-cyclopentenopyridine was added dropwise. After the dropwise addition was completed, the mixture was incubated for 3 hours, and then naturally raised to room temperature for overnight reaction.
[0016] After the reaction, dropwise add methanol 50mL + concentrated hydrochloric acid 160mL + deionized water 200mL mixed solution, control the temperature of the feed liquid during the dropwise addition to not exceed 10°C, and fully stir at this temperature for 10 minutes. Then the li...
Embodiment 2
[0019] The preparation of embodiment 2 cefpirome sulfate
[0020] In the flask, add 550mL of deionized water, 250mL of DMF, 150ml of isopropanol, add 40g of 7-ACP and 80g of AE active ester, then cool to -10°C, and add 30mL of diethylamine dropwise. During the dropwise addition, the temperature does not exceed 0 ℃. After the dropwise addition was complete, stirring was continued for 2 hours. Then the temperature was raised to 15-20° C. for 3 hours, and the reaction solution was extracted three times with 500 mL of dichloromethane. The organic phase was back-extracted with 300 mL of deionized water, and the aqueous phase was combined. The aqueous phase was decolorized with activated carbon, and then quickly filtered through an aluminum oxide column.
[0021] Then add 80 mL of 6mol / L sulfuric acid to control PH=1.5, stir at room temperature for 1 hour, then add 3 L of acetone, stir for 2 hours, filter, wash the filter cake with acetone, and dry to obtain 57 grams of cefpirome ...
Embodiment 3
[0025] 7-ACA is placed in an organic solvent, and the weight ratio of the 7-ACA to the organic solvent N, N-dimethylformamide (DMF) or N, N-dimethylacetamide (DMAC) is 1: 1; Add hexamethyldisilazane and iodotrimethylsilane or trimethylchlorosilane with 1 times the dosage of 7-ACA, and reflux for 2 hours to obtain a brown reaction solution, cool to room temperature, and add 7-ACA dosage dropwise 1 times of trimethyl iodosilane, then dropwise adding 2,3-cyclopentenopyridine of 1 times the amount of 7-ACA, and then acidolysis to obtain 7-ACP;
[0026] The resulting 7-ACP and AE active ester weight ratio are mixed at 1:1 and placed in a mixed solvent where the volume ratio of organic solvent and water is mixed at 1:30, and the pH is adjusted to 1:30 with alkali sodium hydroxide or potassium hydroxide. 7.0, dissolved and acylation reaction occurred, the temperature of acylation reaction was -10°C.
[0027] The obtained cefpirome solution is added into a sulfuric acid solution to c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com