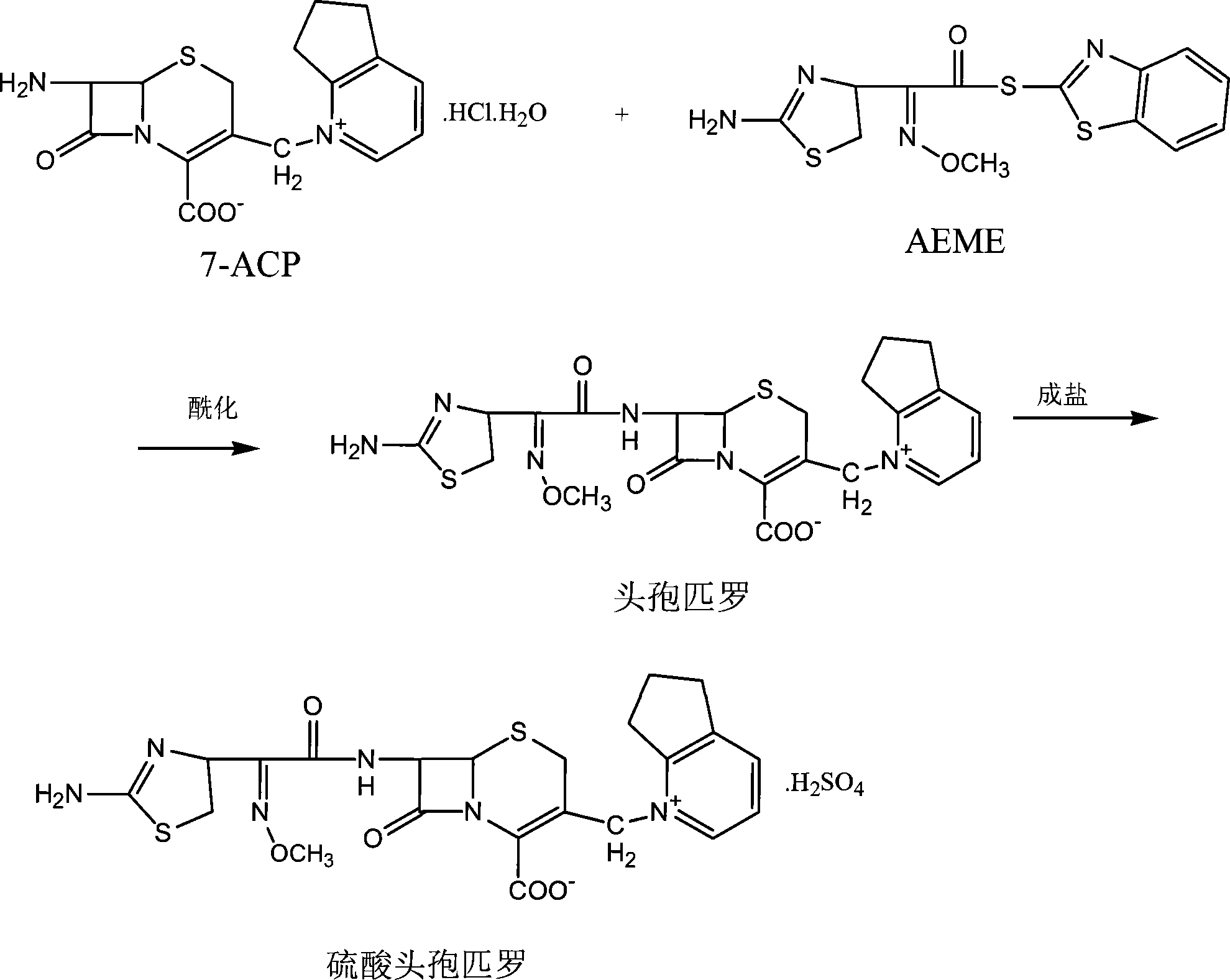
Synthesis process of cefpirome sulfate as antibiotic
A cefpirome sulfate and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems of high raw material cost, harsh reaction conditions, long reaction time, etc., and achieve the effects of stable product quality, simple process conditions, and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 300 ml of deionized water and 150 ml of DMF into a 500 ml four-necked reaction flask, cool down to 0°C to -5°C, add 20 g of 7-ACP and 31.2 g of AEME, and dropwise add 13.5 ml of triethyl ether within 60 minutes The amine adjusts the pH value at 7.0 to 7.8, controls the temperature at 0°C to -5°C, stirs and reacts for 1 hour, then raises the temperature to 15°C to 18°C, reacts for 6 hours, and uses HPLC to detect that the reaction reaches the end point (residual 7 - After the ACP concentration is lower than 1 mg / ml), the reaction solution is extracted three times with 150 ml of dichloromethane each time. After the organic phase is separated, 5 g of activated carbon is added to the aqueous phase, stirred at room temperature for 30 minutes, filtered, and used 50 ml Deionized water washes gac, the solution that merges filtrate and washing gac obtains, after passing through 50 gram gamma-alumina columns (diameter is 2.5 centimeters, length is the glass chromatography colu...
Embodiment 2
[0025] The DMF in the first embodiment is replaced by DMAC, and the result is the same as that in the first embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com