Preparation methods of cefpirome intermediate and cefpirome
A technology for cefpirome and aminocephalosporanic acid, which is applied in the field of drug synthesis, can solve the problems of difficult treatment and recovery of three wastes, cumbersome post-processing operations, waste of iodine cost, and the like, achieves simplified treatment and recovery, saves cumbersome processes and waste of raw materials, The effect of saving crystallization solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
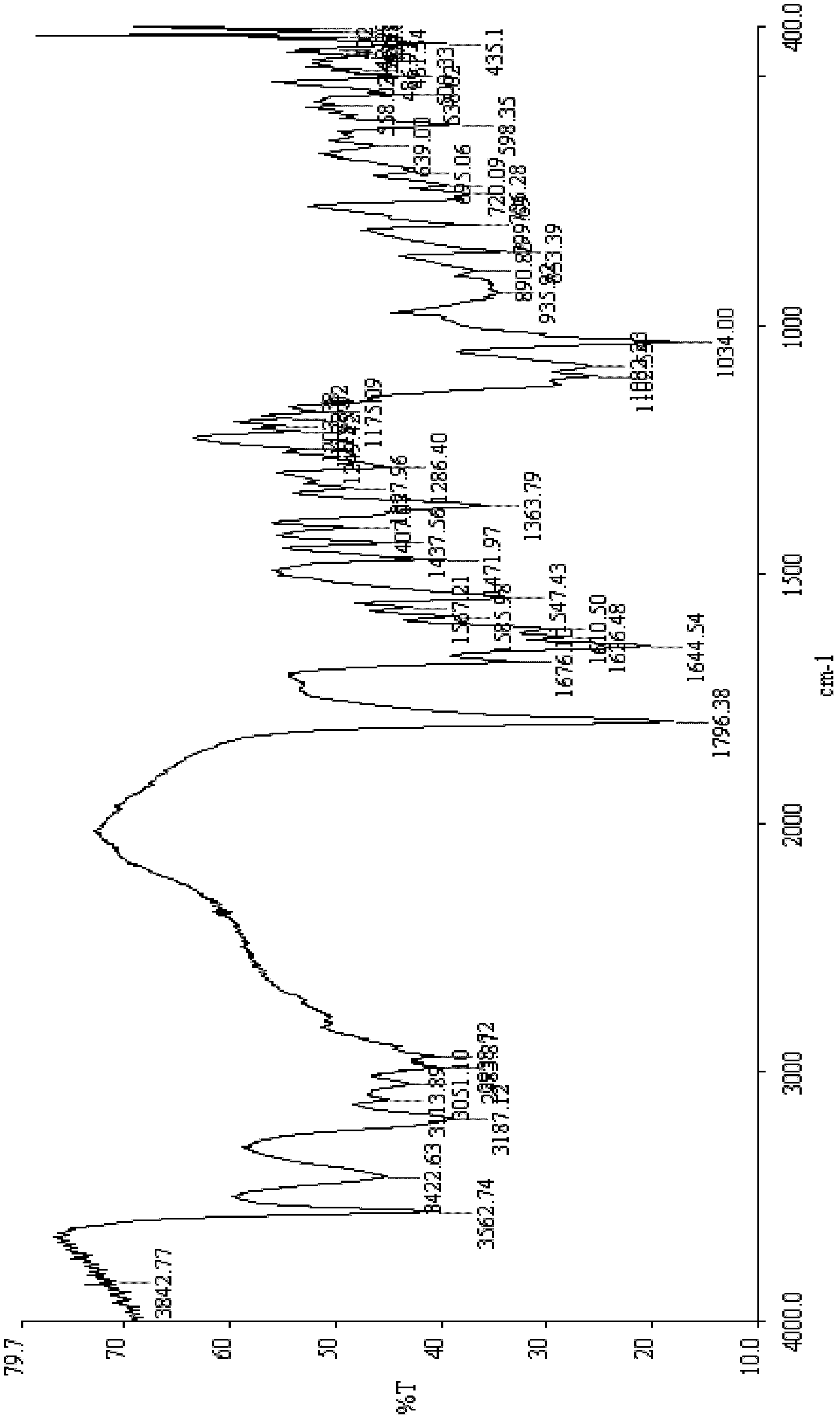
[0050] [embodiment 1] the preparation of cefpirome intermediate (formula 1, wherein HX is HCl) monohydrate
[0051] (1) 40g (0.147mol) of 7-aminocephalosporanic acid (formula 4), 150ml of dichloromethane, and 48ml (0.23mol) of hexamethyldisilazane (HMDS) were placed in a reaction flask, heated to reflux for 8 hours, Add 29ml (0.18mol) of N,N-diethylaniline (0.18mol) and TMSI (40.8g, 0.204mol) under ice-cooling, react at room temperature for 3 hours, cool down to 0-5°C, add 24ml of 2,3-cyclopentenopyridine ( 0.182mol), temperature controlled at 5-10°C and stirred for 5hr to obtain a solution of the compound of formula 5;
[0052] (2) Add 40ml of methanol dropwise to the solution of the compound of formula 5 prepared above, react for 10mins, add 10ml (0.21mol) of hydrogen peroxide with a concentration of 50wt%, 120ml of concentrated hydrochloric acid, and 120ml of water, stir until the solids are all dissolved, and let stand for stratification. Add 550ml of acetone to the water...
Embodiment 2
[0053] [embodiment 2] the preparation of cefpirome sulfate
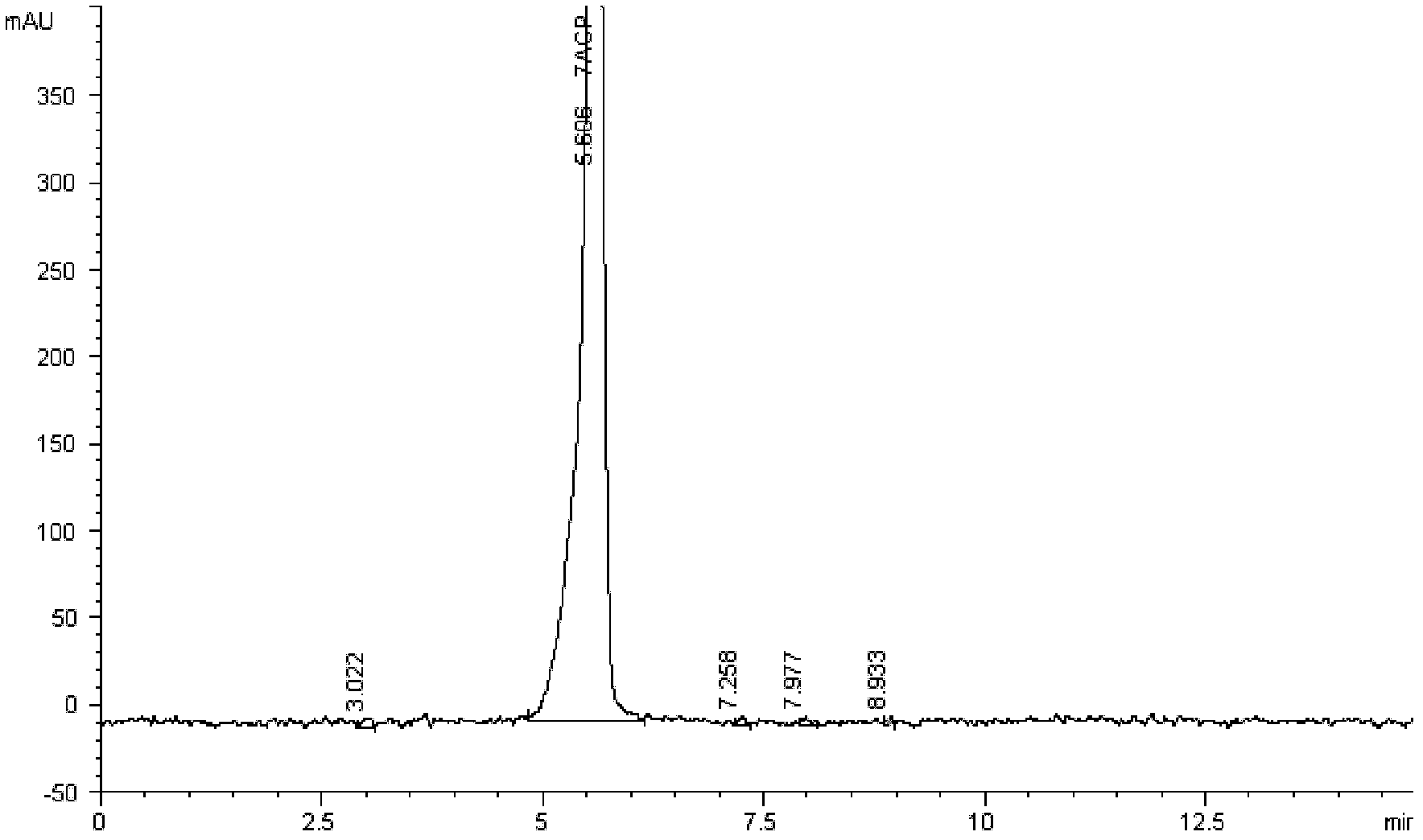
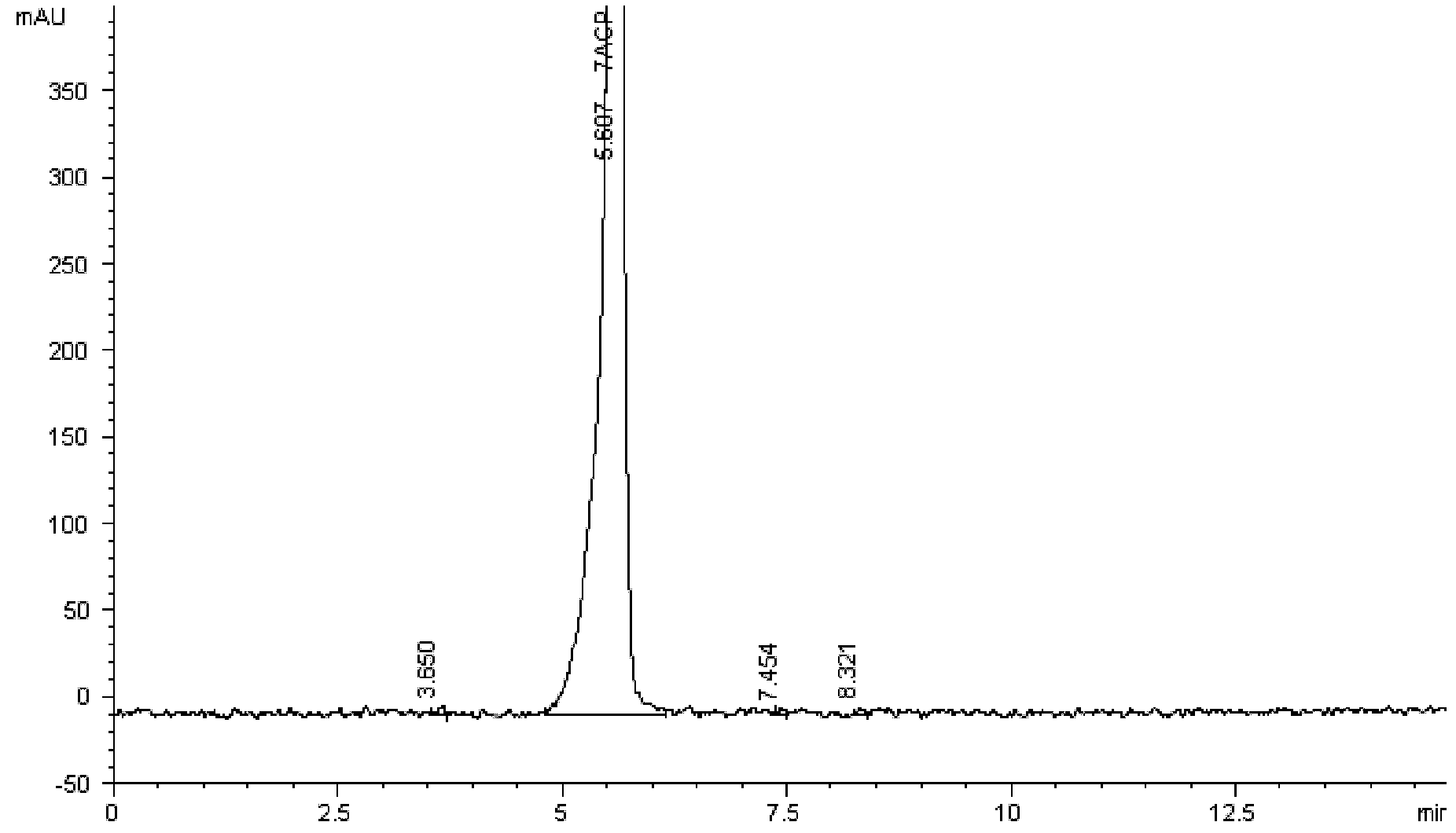
[0054] N, N-dimethylformamide 350ml (DMF), water 125ml, cefpirome intermediate monohydrochloride monohydrate 40g (prepared by Example 1), AE-active ester 42g is placed in the reaction flask, cooling To 0~5℃, add 13.5ml of triethylamine dropwise, keep stirring for 4hr after adding, add 480ml of dichloromethane, stir for 30mins, let it stand for stratification, add 10g of activated carbon to the water phase for decolorization, filter, and the concentration of the filtrate is 40wt% Adjust the pH to 1.2-1.5 with sulfuric acid, add 2500ml of acetone at room temperature, cool to 0-5°C and stir for 1 hr, filter, wash the filter cake with 200ml of acetone, and dry in vacuo to obtain 56.6g of white solid, content 86.4%, HPLC purity > 99%, That is cefpirome sulfate, the molar yield is 92.5%.
Embodiment 3
[0055] [embodiment 3] the synthesis of cefpirome intermediate (formula 1, wherein HX is HI) monohydrate
[0056] Step (1) is as described in Example 1, the difference is: add methanol 120ml and water 80ml dropwise to the solution of the compound of formula 5, stir for 2hr after adding, filter, filter cake is washed with methanol 150ml, and vacuum-dried to obtain a White product 64.9g (molar yield 92.1%), content 69.1%, purity > 98% (HPLC), KF: 3.9%, iodide ion content: 26.9%, be cefpirome intermediate hydroiodide monohydrate .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com