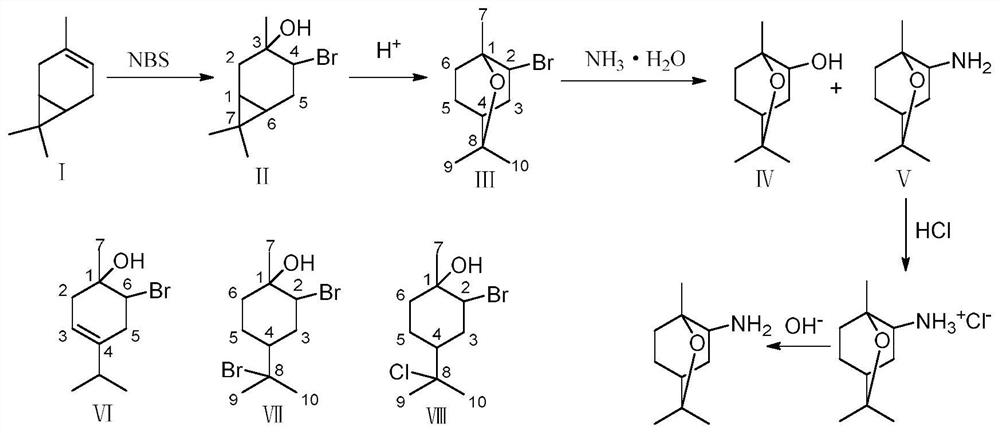
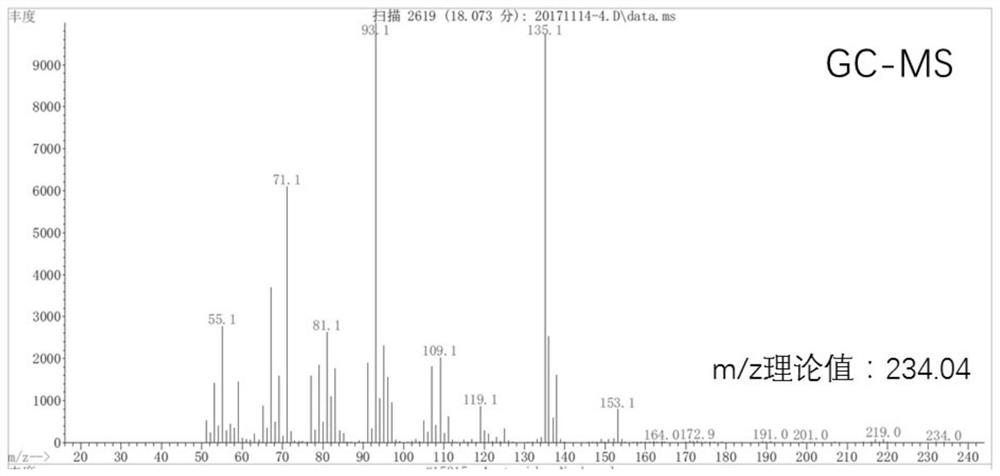
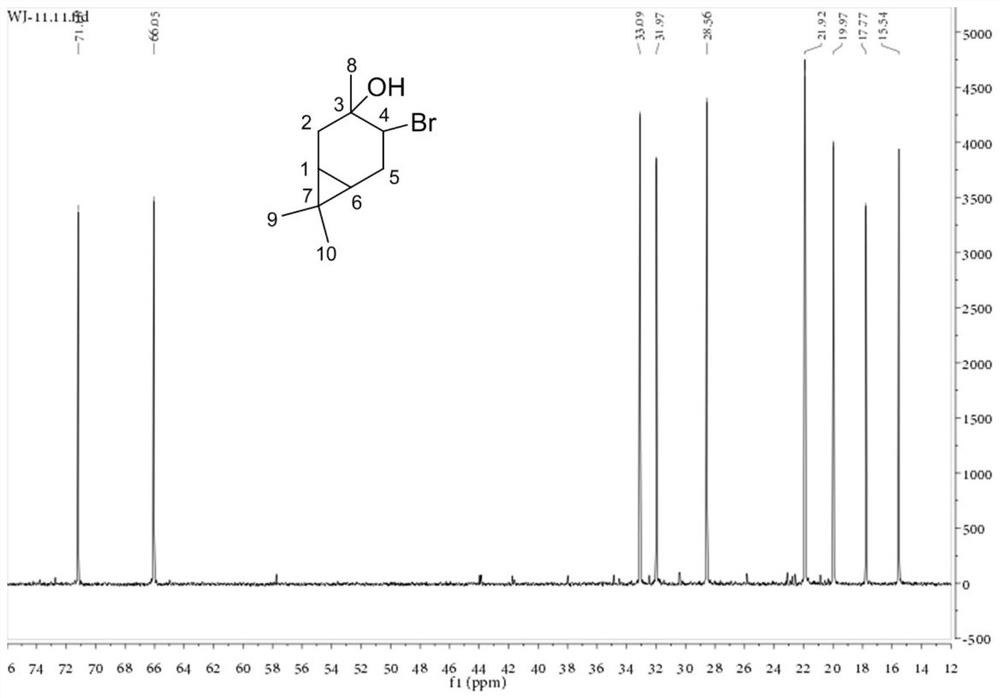
Method for preparing 1,8-cineole derivative from 3-carene
A technology for eucalyptol and derivatives, which is applied in the field of preparing 1,8-cineole derivatives from 3-carene, can solve the problems of rare amine derivatives, and achieve abundant natural reserves, low price and high yield selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of product A with 3-hydroxy-4-bromo-carbene as main component
[0041] 10.8g 3-carene, 8g CaCO 3 , 40 mL of water, 80 mL of 1,4-dioxane and 20 g of NBS were sequentially added to the reaction flask, and stirred at room temperature for 2 h; the reaction solution was transferred to 200 mL of water, filtered to remove the precipitate, and extracted with ethyl acetate (200 mL × 3 times), The aqueous layer was discarded; the organic layer was washed with 10% sodium thiosulfate-aqueous solution (200 mL×3 times), the aqueous layer was discarded, and the organic layer was dried by adding anhydrous sodium sulfate; the desiccant was removed by filtration, and the solvent was recovered by rotary evaporation to obtain the product A15.2g, wherein the GC content of compound II is 71.8%.
Embodiment 2
[0042] Example 2 Preparation of 2-bromo-1,8-cineole
[0043] Add 1 g of product A obtained in Example 1 and 5 mL of chloroform into the reaction flask, turn on and stir, add 0.94 g of trimethylbromosilane, and stir at 20°C for 1 h; the solvent is recovered by rotary evaporation to obtain product B, wherein compound III, The GC contents of compound VI and compound VII are: 42.95%, 16.18% and 13.14%, respectively.
Embodiment 3
[0044] Example 3 Preparation of 2-bromo-1,8-cineole
[0045] Add 1 g of product A obtained in Example 1 and 5 mL of chloroform into the reaction flask, turn on and stir, add 0.47 g of trimethylbromosilane, and stir at 20°C for 1 h; the solvent is recovered by rotary evaporation to obtain product B, wherein compound III, The GC contents of compound VI and compound VII are: 36.61%, 14.68% and 6.53%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com