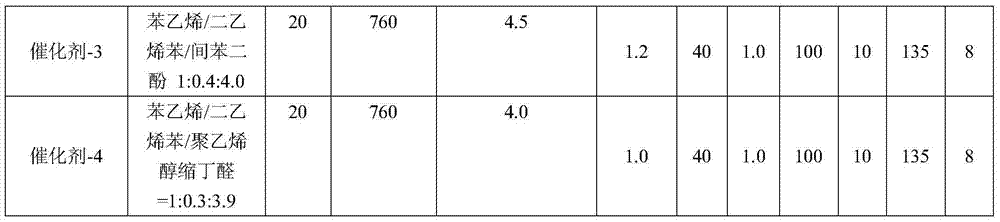
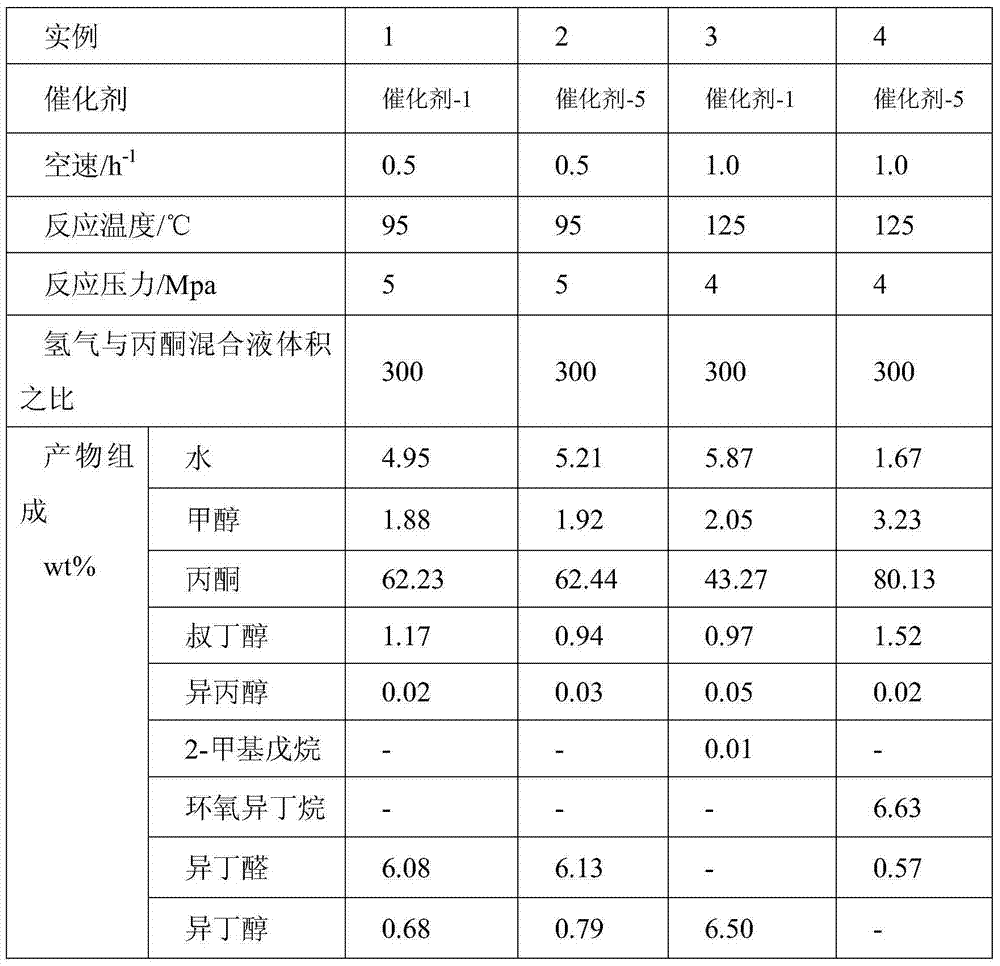
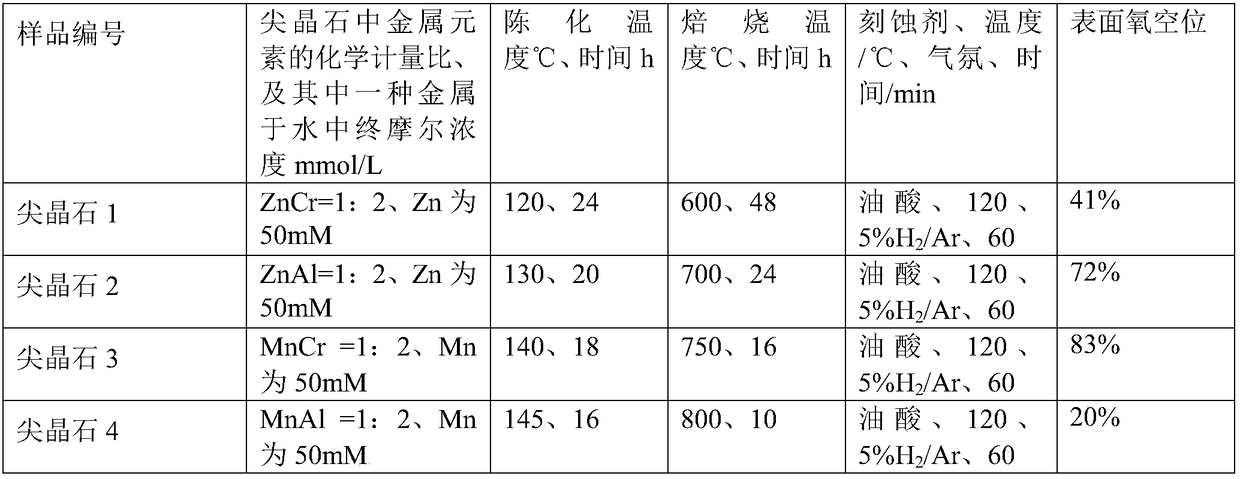
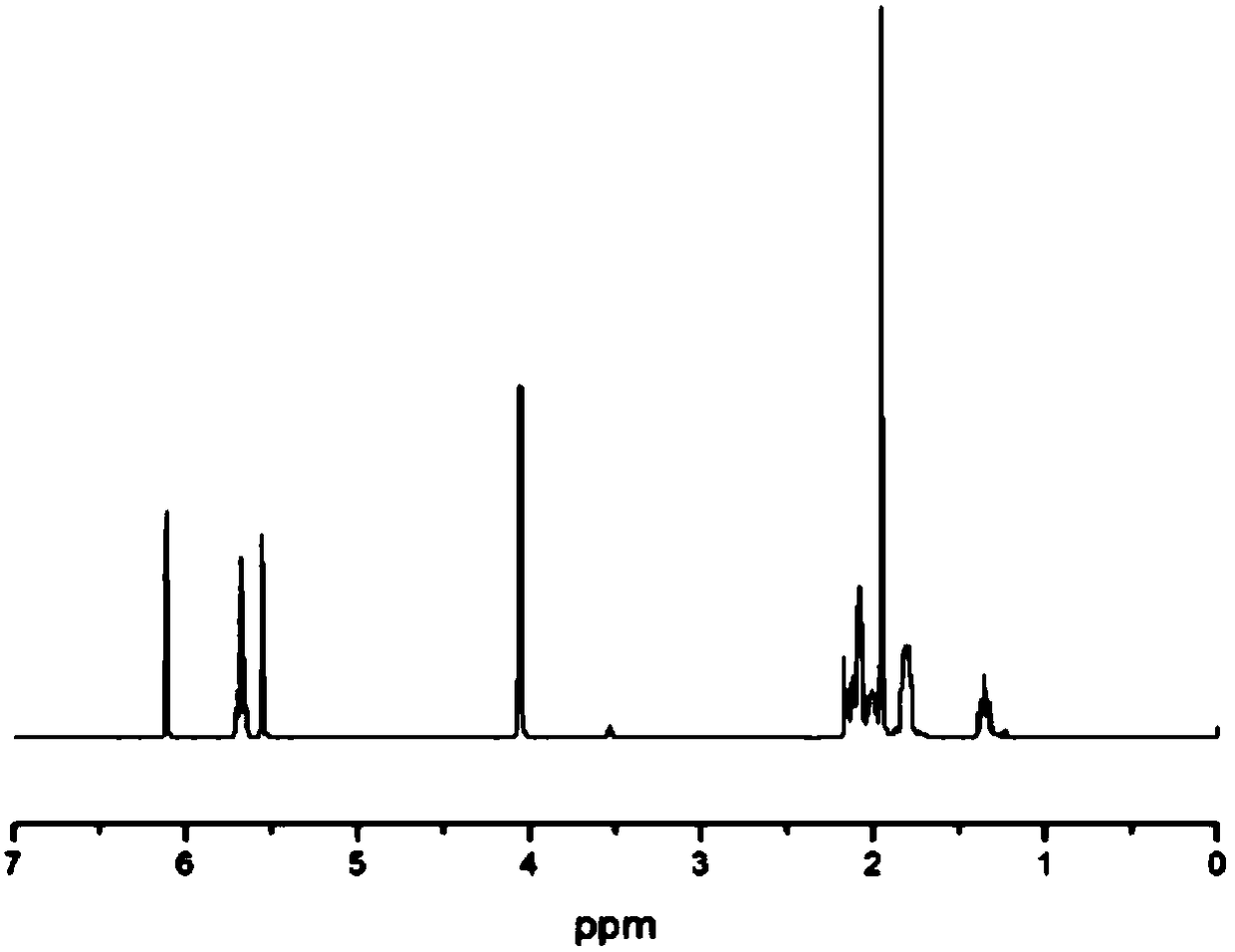
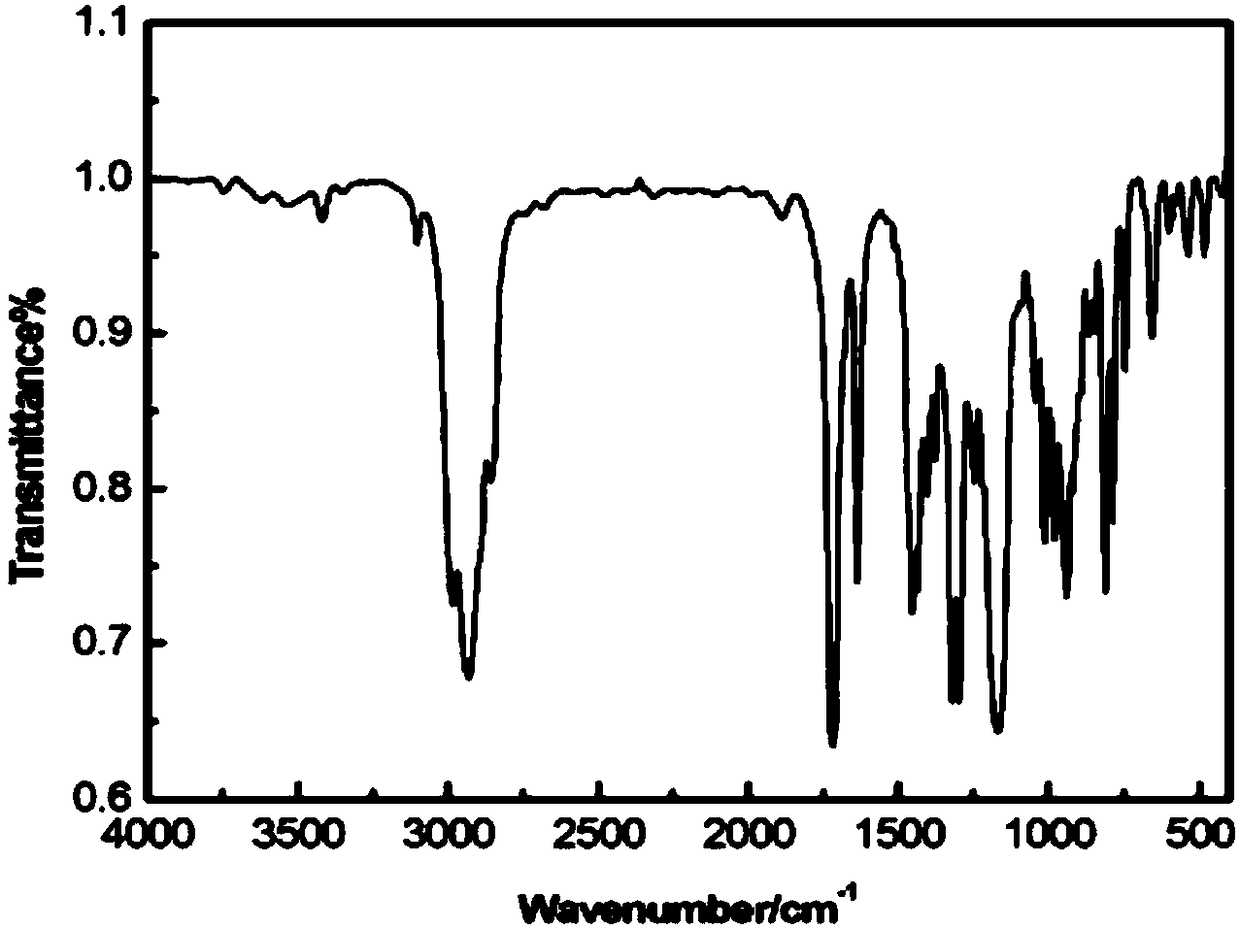
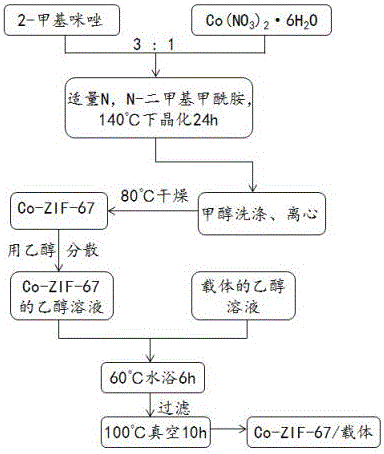
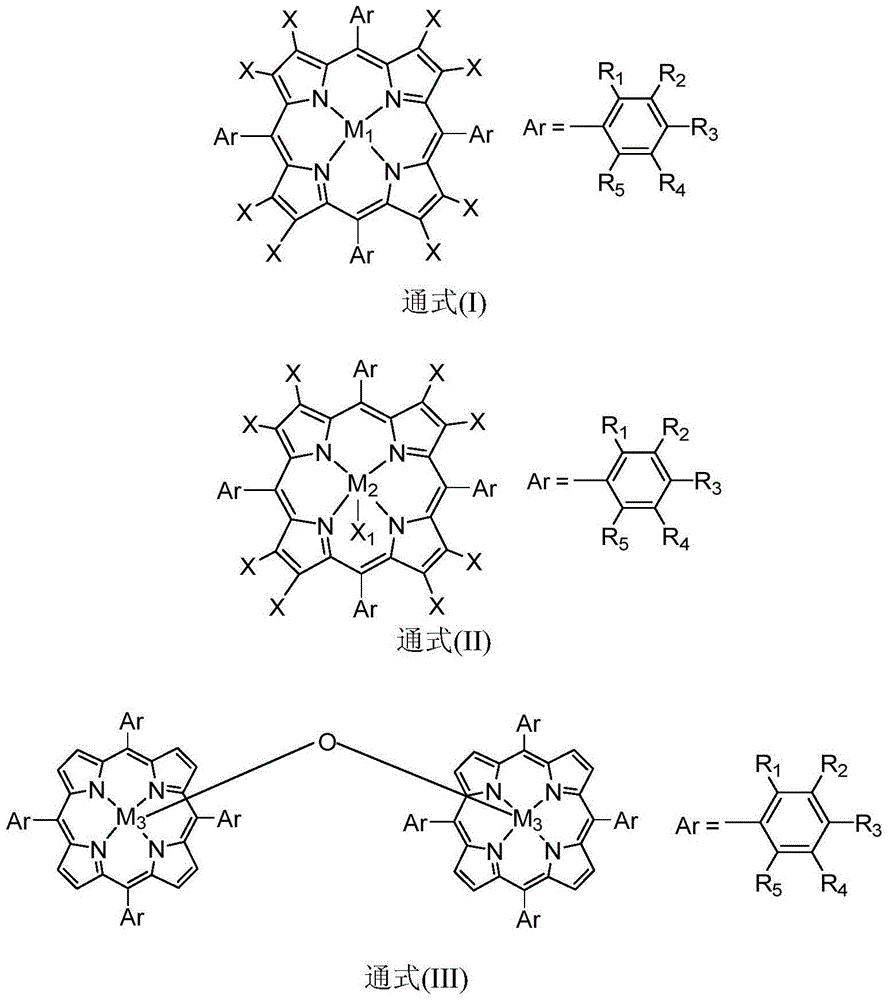
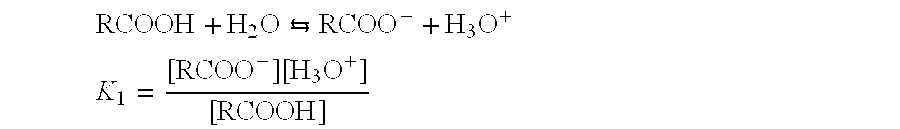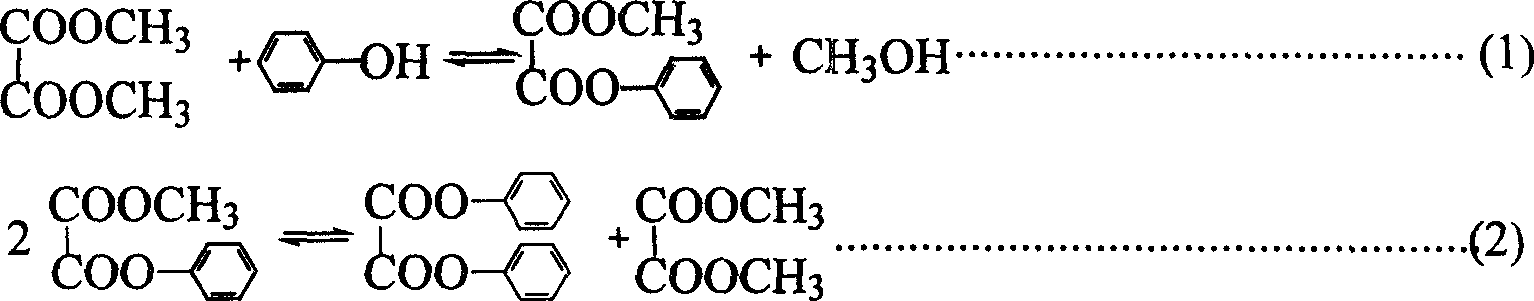Patents
Literature
107results about How to "High product selectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
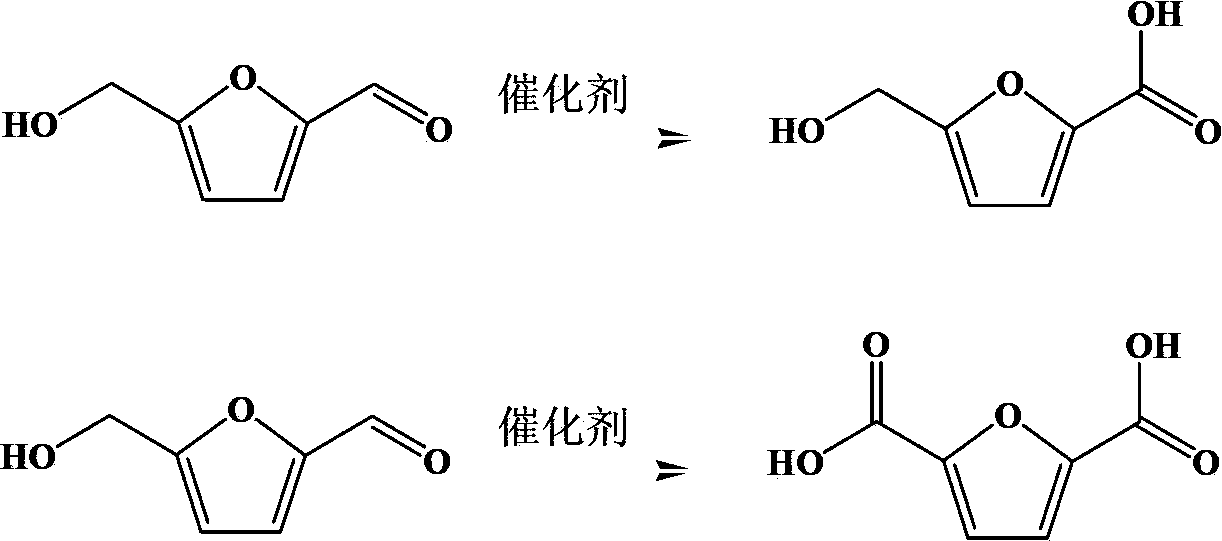
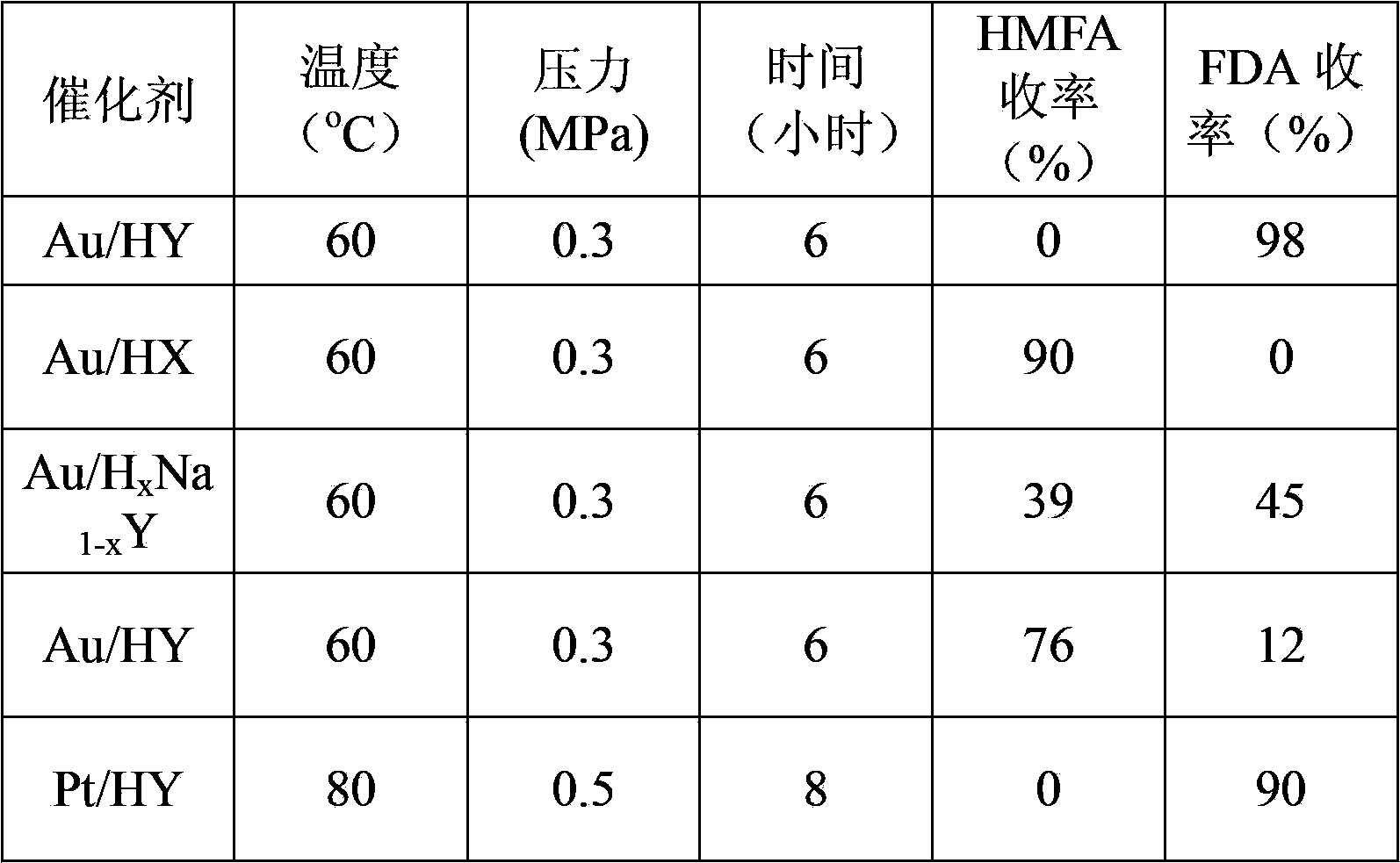
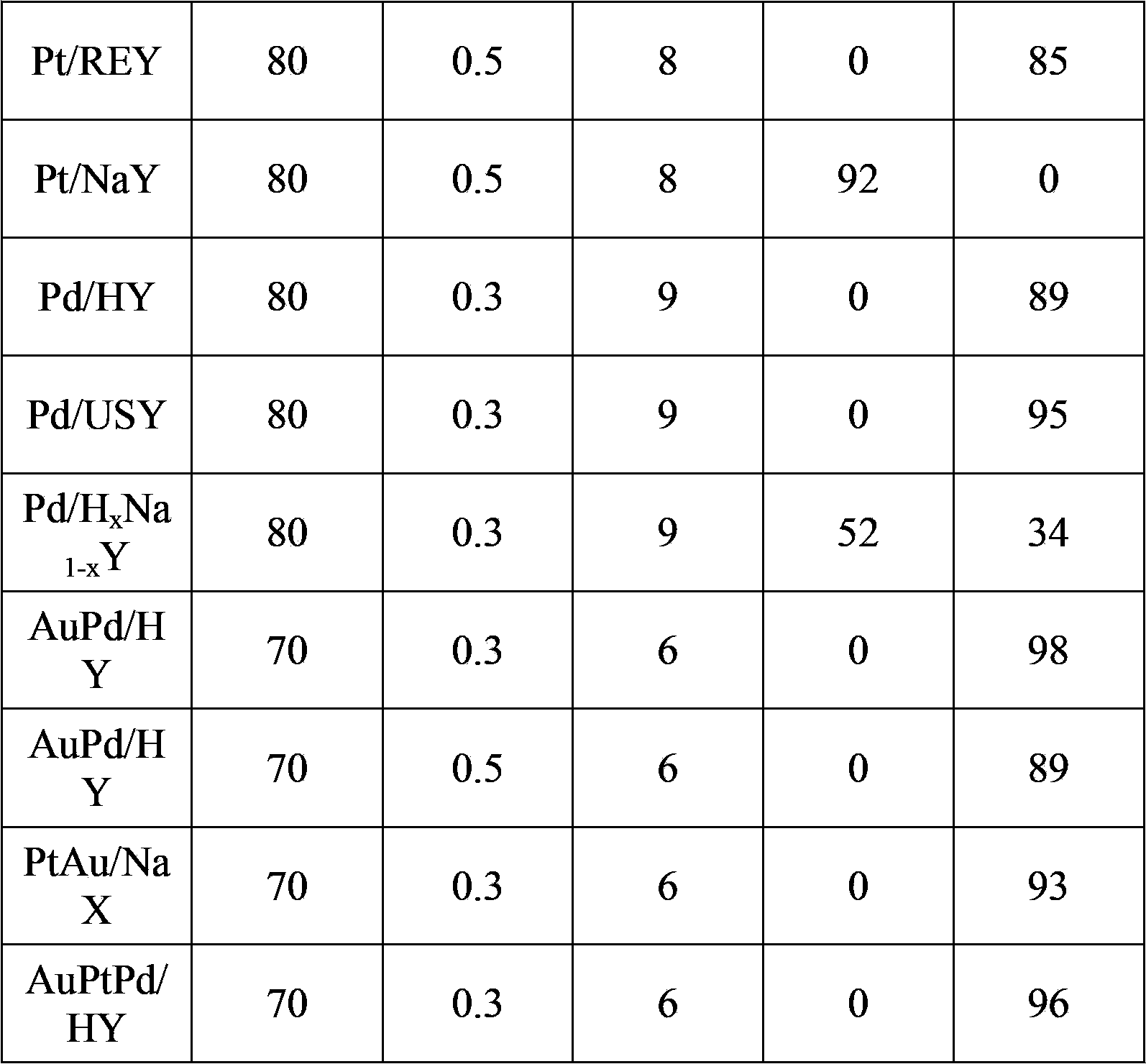
Preparation method of 5-hydroxymethyl furoic acid and 2,5-furandicarboxylic acid
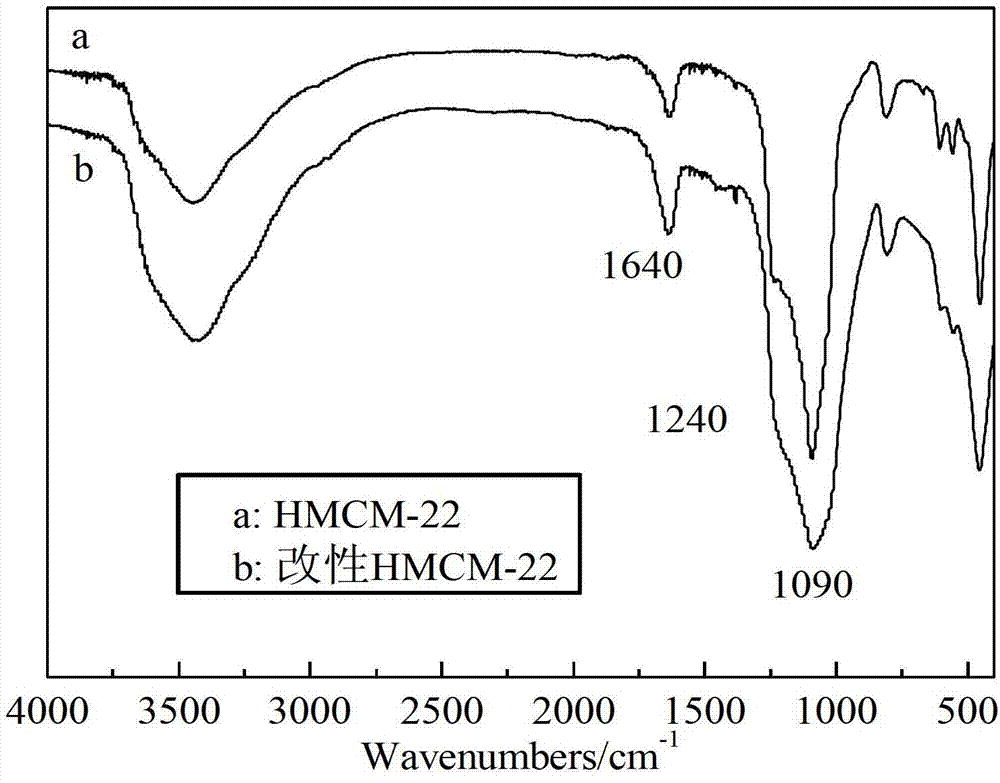
ActiveCN103626726AEasy to makeModification operation is simpleOrganic chemistryMolecular sieve catalystsHydroxymethylfurfuralHydroxymethyl
The invention discloses a preparation method of 5-hydroxymethyl furoic acid and 2,5-furandicarboxylic acid by selective oxidation of 5-hydroxymethylfurfural. The method comprises the following steps: taking a precious metal loaded acidic carrier as the catalyst, under mild conditions (temperature is in a range of 25 to 100 DEG C, and the oxygen pressure is in a range of 0.1 to 3.0 MPa), and modulating the temperature, pressure and reaction time so as to rapidly and high-efficiently oxidize 5-hydroxymethylfurfural to generate 5-hydroxymethyl furoic acid or 2,5-furandicarboxylic acid. The conversion rate of 5-hydroxymethylfurfural can reach 100%, the selectivity of 5-hydroxymethyl furoic acid reaches 98%, and the selectivity of 2,5-furandicarboxylic acid reaches 99%. The method has the advantages of high efficiency and environment-friendliness, and the products have a big application value and a wide application prospect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
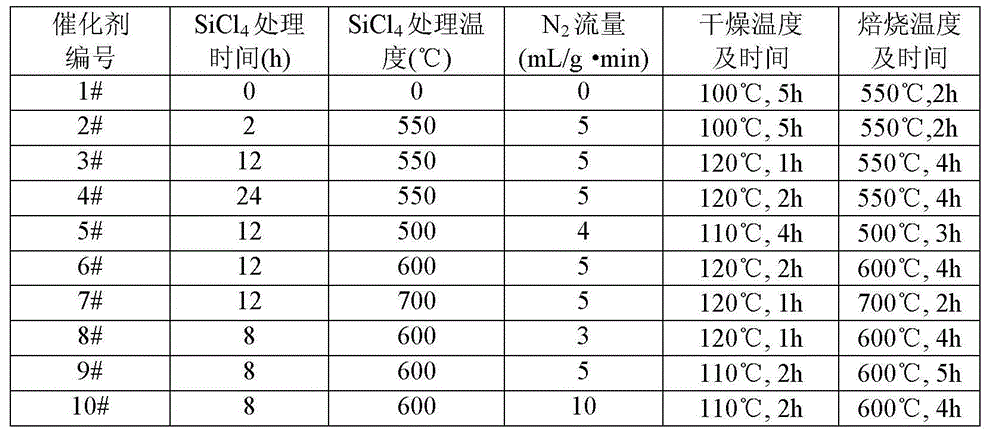
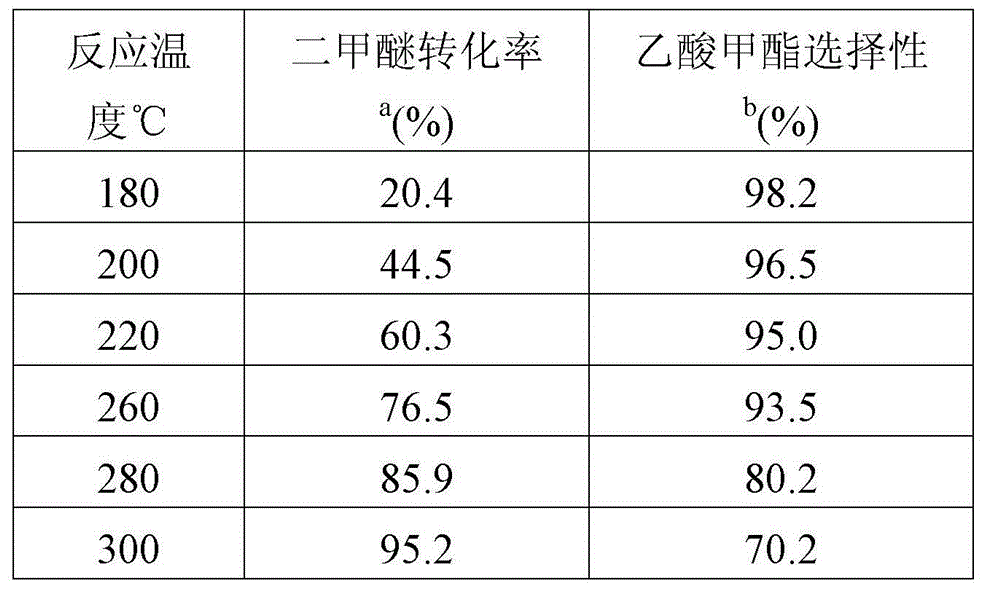
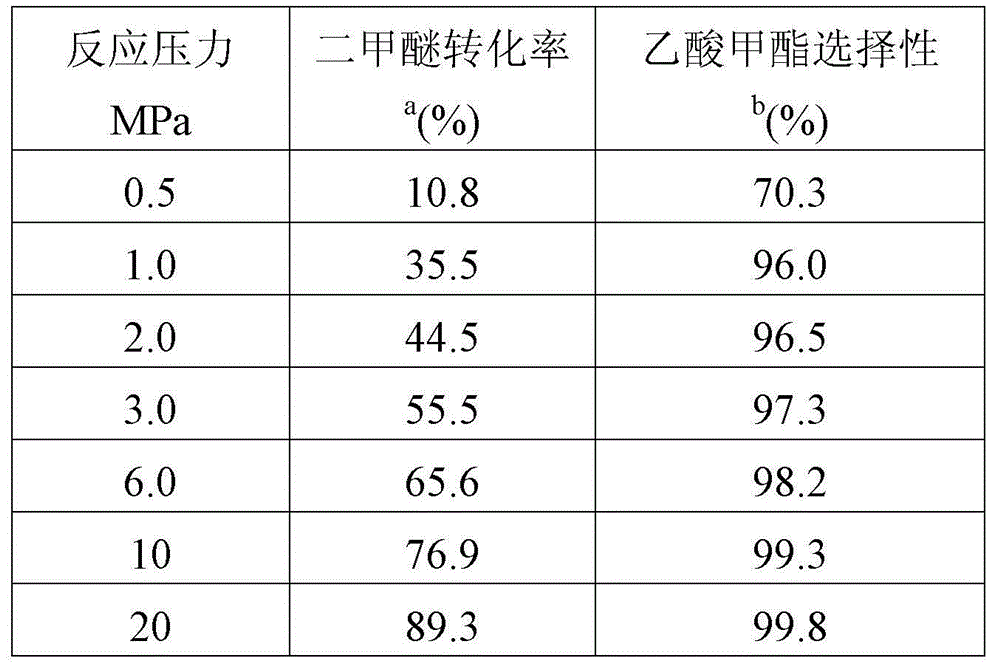
Catalyst used for preparing methyl acetate through dimethyl ether carbonylation, preparation method and application thereof
ActiveCN104689845AHigh product selectivityLong catalyst lifeMolecular sieve catalystsPreparation by carbon monoxide or formate reactionProtein carbonylMolecular sieve
The invention provides a catalyst used for preparing methyl acetate through dimethyl ether carbonylation, a preparation method and an application thereof, more specifically, the invention provides the catalyst used for preparing methyl acetate through dimethyl ether carbonylation, the catalyst contains a silicon tetrachloride steam dealuminated hydrogen-mordenite molecular sieve, and silicon and aluminum atomic ratio is 3: 1-20: 1. According to the invention, silicon tetrachloride steam is contacted to mordenite for effecting acid site in 12-membered ring tunnel without influence of acid site in 8-membered ring tunnel, aluminum in 12-membered ring tunnel is selectively removed, and acid site in 12-membered ring tunnel is kept, so that life and selectivity of catalyst in a reaction for preparing methyl acetate through dimethyl ether carbonylation are increased, and reaction yield is increased.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
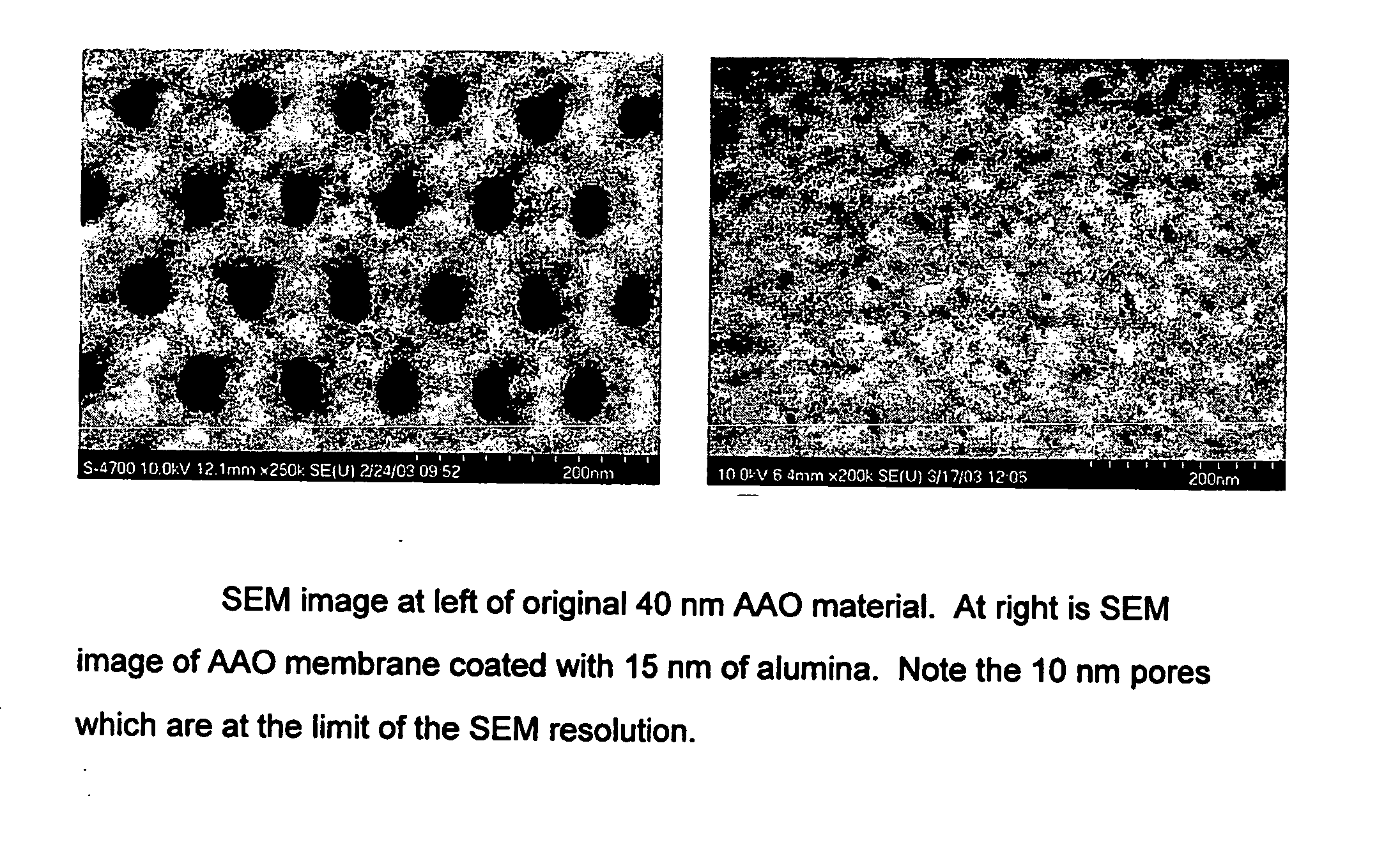
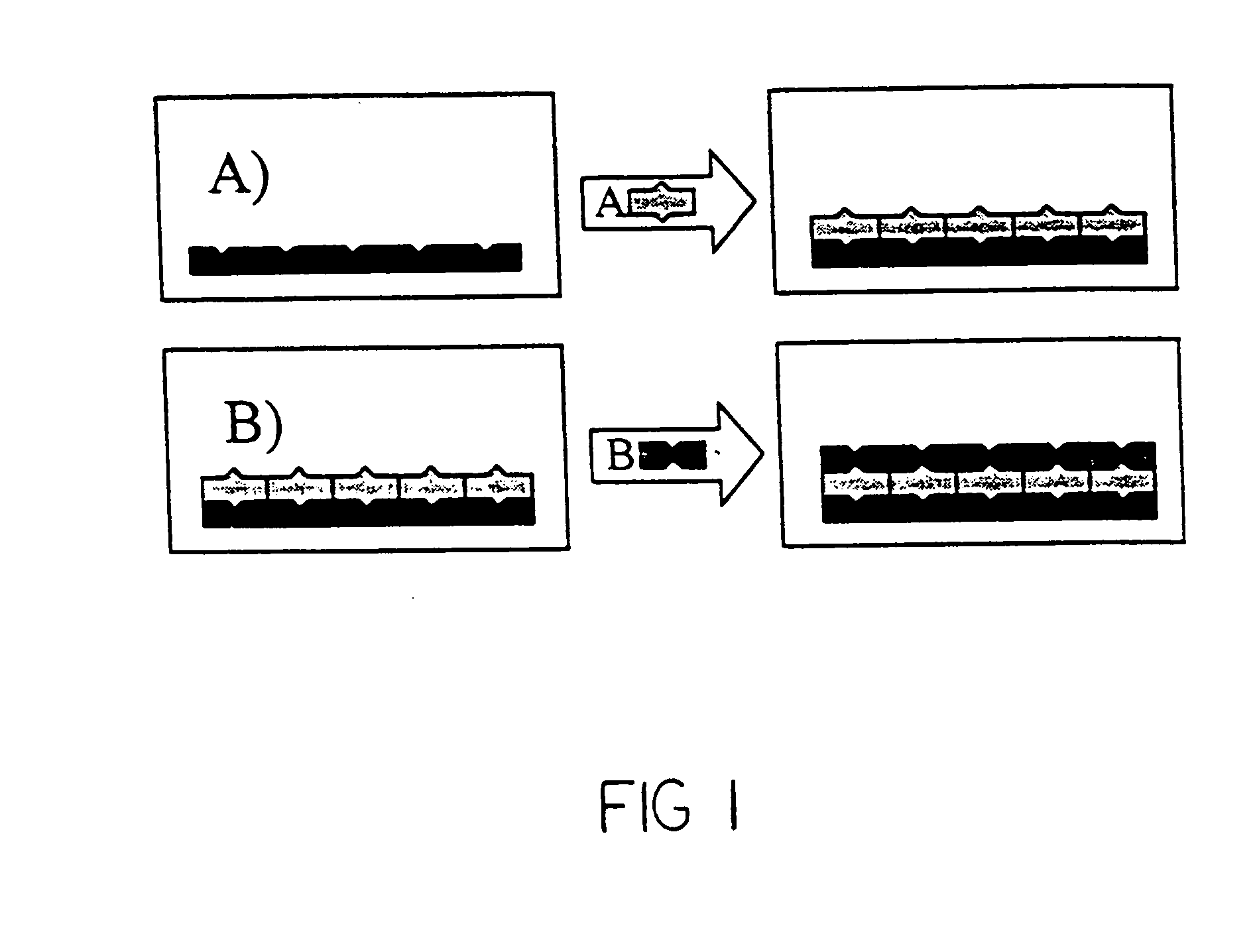
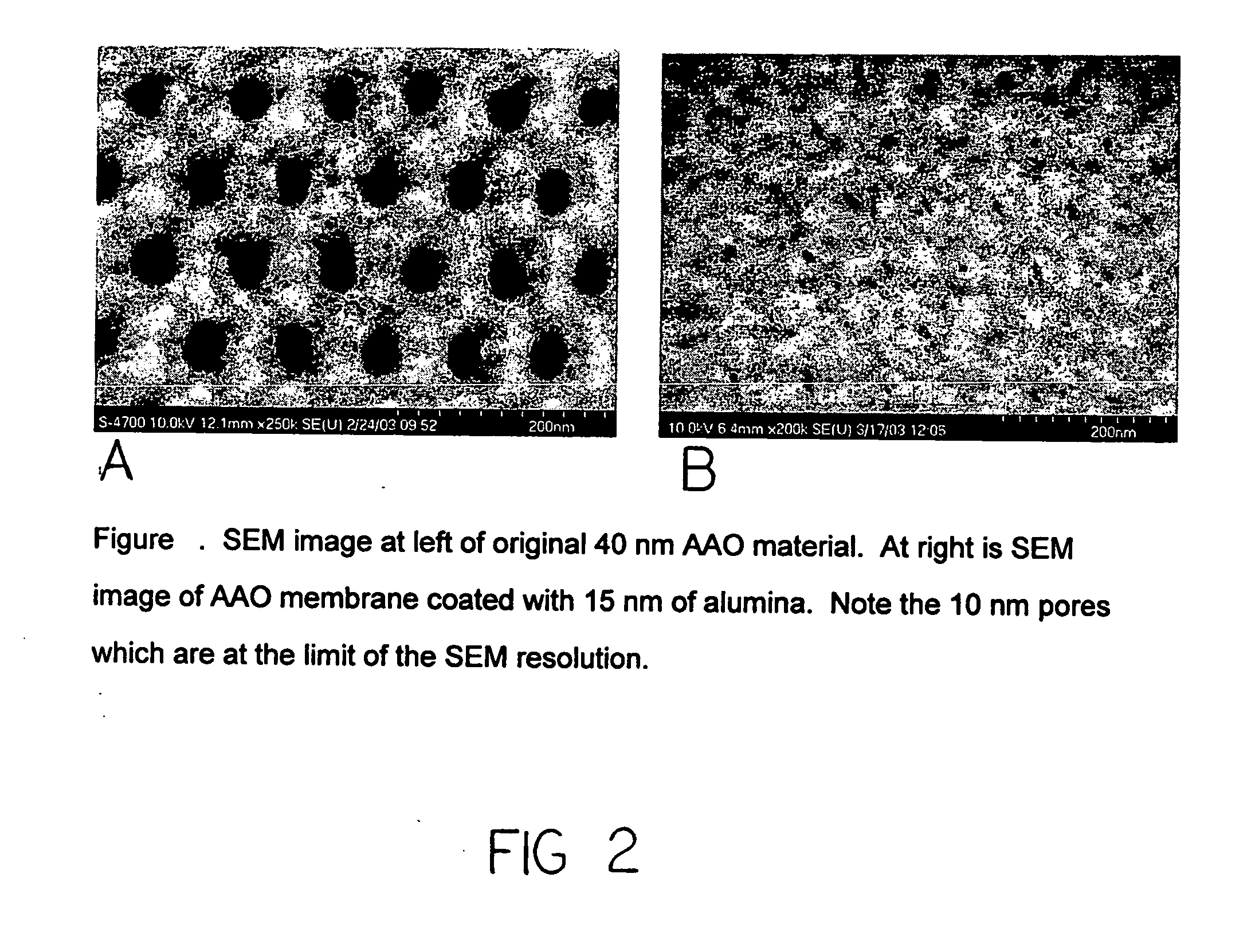
Catalytic nanoporous membranes
InactiveUS20050065028A1Unique catalyst environmentUniform coatingNanotechOther chemical processesPartial oxidationNanoporous membrane
A nanoporous catalytic membrane which displays several unique features including pores which can go through the entire thickness of the membrane. The membrane has a higher catalytic and product selectivity than conventional catalysts. Anodic aluminum oxide (AAO) membranes serve as the catalyst substrate. This substrate is then subjected to Atomic Layer Deposition (ALD), which allows the controlled narrowing of the pores from 40 nm to 10 nm in the substrate by deposition of a preparatory material. Subsequent deposition of a catalytic layer on the inner surfaces of the pores reduces pore sizes to less than 10 nm and allows for a higher degree of reaction selectivity. The small pore sizes allow control over which molecules enter the pores, and the flow-through feature can allow for partial oxidation of reactant species as opposed to complete oxidation. A nanoporous separation membrane, produced by ALD is also provided for use in gaseous and liquid separations. The membrane has a high flow rate of material with 100% selectivity.
Owner:UCHICAGO ARGONNE LLC
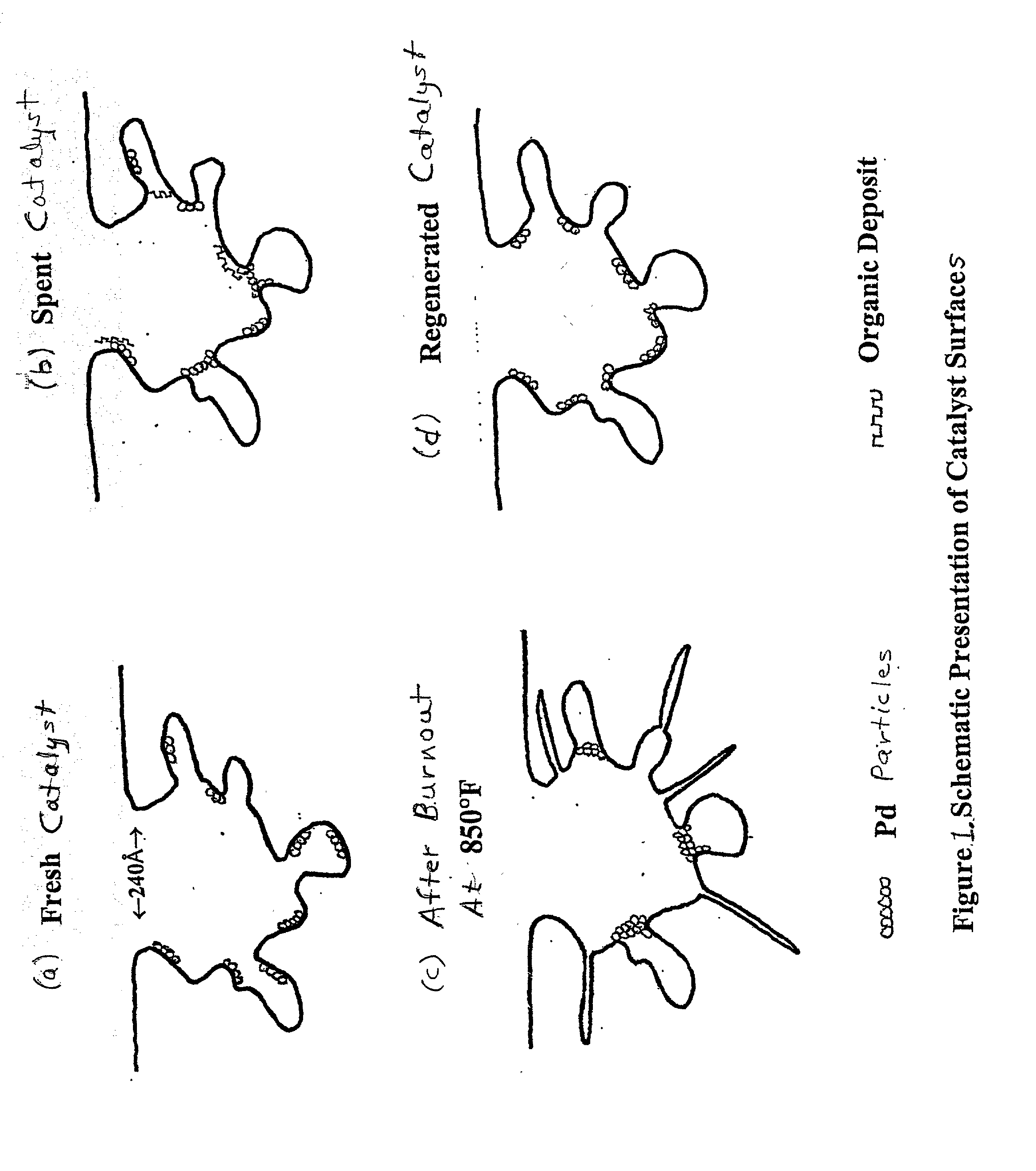
Regeneration of used supported noble metal catalysts
InactiveUS20020115554A1Easy to cleanHigh activityHydrogen peroxideOther chemical processesPtru catalystPalladium catalyst
A method for regenerating used supported noble metal catalysts, which method includes solvent cleaning the used catalyst by contact with a suitable organic liquid cleaning solvent such as alcohols, ketones and such to remove organic deposits from the catalyst, followed by drying and calcining at elevated temperature to remove any remaining organic deposits from the catalyst, then treating the catalyst with an organo-metallic complex forming agent having ionization constant pK1 greater than about 2.5, such as glycolic acid and the like. The organic-metallic complex forming agent acts to break down large clusters of noble metal particles such as palladium (Pd) and redistributes the metal particles on the catalyst support such as alumina (Al2O3) in the same or other larger pores, so as to increase catalyst surface area and catalytic activity to provide a catalytic activity level at least 80% or even exceeding that of the fresh catalyst. This regeneration method is particularly useful for regenerating used supported palladium catalysts utilized for hydrogenation of ethyl anthraquinone (EAQ) for producing hydrogen peroxide (H2O2) product.
Owner:POROCEL INT LLC +1
High efficiency processes for olefins, alkynes, and hydrogen co-production from light hydrocarbons such as methane
InactiveUS20140058149A1Process environmental protectionIncreases overall C selectivityThermal non-catalytic crackingHydrocarbon by hydrogenationMethanationOxygen
High efficiency processes for producing olefins, alkynes, and hydrogen co-production from light hydrocarbons are disclosed. In one version, the method includes the steps of combusting hydrogen and oxygen in a combustion zone of a pyrolytic reactor to create a combustion gas stream, transitioning a velocity of the combustion gas stream from subsonic to supersonic in an expansion zone of the pyrolytic reactor, injecting a light hydrocarbon into the supersonic combustion gas stream to create a mixed stream including the light hydrocarbon, transitioning the velocity of the mixed stream from supersonic to subsonic in a reaction zone of the pyrolytic reactor to produce acetylene, and catalytically hydrogenating the acetylene in a hydrogenation zone to produce ethylene. In certain embodiments, the carbon efficiency is improved using methanation techniques.
Owner:UOP LLC
Catalyst for preparing 1,4-cyclohexane dimethanol from hydrogenation of 1,4-cyclo hexane diformic acid
InactiveCN1911504AHigh product selectivityHigh reactivityOrganic compound preparationPreparation by hydrogenationActive componentHexane
The catalyst for hydrogenating 1, 4-cyclohexane diformic acid to prepare 1, 4-cyclohexane dimethanol consists of Al2O3 carrier and supported active components of metal Ru and Sn in the molar ratio of 1 to 0.5-2.0 and accounting for 5-20 wt%, and has grain size of 80-200 mesh. Compared with available technology, the catalyst of the present invention has the advantages of proper catalyst carrier, no use of noble metal Pt and Re, ideal activity and target product selectivity, low cost, etc.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Light alkane dehydrogenation catalyst with good hydrothermal stability and preparation method thereof
InactiveCN104128175AImproves hydration resistanceAvoid chalkingHydrocarbonsMetal/metal-oxides/metal-hydroxide catalystsAlkaneDehydrogenation
The invention relates to a light alkane dehydrogenation catalyst with good hydrothermal stability and a preparation method thereof. The catalyst comprises the following components, by weight, 5-40% of Cr2O3, 30-85% of Al2O3, 0.1-30% of MO and 0.5-5% of Me2O, wherein M is Mg, Zn or a mixture of Mg and Zn; and Me is one of alkalis or a mixture of more than two of alkalis. The light alkane dehydrogenation catalyst has advantages of good hydrothermal stability, high activity and high selectivity, and is used in a dehydrogenation reaction of C3-C5 alkane.
Owner:YANTAI UNIV
Method for preparing high-purity methyl isobutyl ketone from industrial by-product acetone waste liquid
ActiveCN103936574AAvoid wastage of raw materialsHigh product selectivityOrganic compound preparationCarbonyl compound preparationChemistryHomogeneous catalysis
The invention discloses a method for preparing high-purity methyl isobutyl ketone from an industrial by-product acetone waste liquid. According to the method, industrial byproduct acetone waste liquid, which contains more impurities and is difficult to separate, and hydrogen directly pass through a fixed bed tubular reactor filled with a heterogeneous catalyst, thereby converting acetone and difficultly separated impurities into MIBK and substances which are easily separated from the product; the reaction products are purified by distillation to obtain high-purity methyl isobutyl ketone. By virtue of the technology disclosed by the invention, a problem of a complex separation of acetone from impurities is solved, the cost for separating raw materials and the acetone circulation cost are greatly reduced, and the economical benefits of the device are greatly improved by converting the by-product acetone waste liquid into an MIBK product with high added value.
Owner:WANHUA CHEM GRP
Preparing method for loaded titania catalyst of ester interchange synthetic phenyl ester oxalate
ActiveCN1583246AMild operating conditionsSimple processOrganic compound preparationCarboxylic acid esters preparationOxalatePolymer science
A carried TiO2 catalyst used for synthesizing the phenyl oxalate used to prepare diphenyl carbonate (DPC) by ester exchange is prepared through preparing the dipping liquid, pretreating carrier, mixing it with said dipping liquid, hydrolyzing, drying and calcining. It has high activity and no pollution.
Owner:TIANJIN UNIV
Catalyst and method for direct conversion of synthesis gas for preparation of low carbon olefins
ActiveCN108144643AHigh product selectivityReduce energy consumption and costsHydrocarbon from carbon oxidesMolecular sieve catalystsChemistryMolecular sieve
The invention belongs to direct preparation of low carbon olefins from synthesis gas, and particularly relates to a catalyst and a method for direct conversion of the synthesis gas for preparation ofthe low carbon olefins. According to the method, the synthesis gas is adopted as a reaction raw material, a conversion reaction is carried out on a fixed bed or a moving bed, and the catalyst is a A+Bcomposite catalyst which is compounded by mixing a catalyst A and a catalyst B mechanically, wherein the active ingredient of the catalyst A is active metal oxide, and the catalyst B is one or two ormore selected from a molecular sieve of a CHA and AEI structure or a metal-modified CHA and / or AEI molecular sieve; a spacing between the geometric center of particles of the active metal oxide of the catalyst A and the geometric center of particles of the catalyst B is 5[mu]m-40 mm, a spacing between axial centers of particles is preferably 100 [mu]m-5 mm, more preferably 200 [mu]m-4 mm, and theweight ratio of the active ingredient in the catalyst A to the catalyst B is in a range of 0.1-20, preferably 0.3-5.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Catalytic nanoporous membranes
InactiveUS20100075827A1Unique catalyst environmentUniform coatingOther chemical processesCatalyst activation/preparationPartial oxidationGas phase
A nanoporous catalytic membrane which displays several unique features Including pores which can go through the entire thickness of the membrane. The membrane has a higher catalytic and product selectivity than conventional catalysts. Anodic aluminum oxide (AAO) membranes serve as the catalyst substrate. This substrate is then subjected to Atomic Layer Deposition (ALD), which allows the controlled narrowing of the pores from 40 nm to 10 nm in the substrate by deposition of a preparatory material. Subsequent deposition of a catalytic layer on the inner surfaces of the pores reduces pore sizes to less than 10 nm and allows for a higher degree of reaction selectivity. The small pore sizes allow control over which molecules enter the pores, and the flow-through feature can allow for partial oxidation of reactant species as opposed to complete oxidation. A nanoporous separation membrane, produced by ALD is also provided for use in gaseous and liquid separations. The membrane has a high flow rate of material with 100% selectivity. Also provided is a method for producing a catalytic membrane having flow-through pores and discreet catalytic clusters adhering to the inside surfaces of the pores.
Owner:UCHICAGO ARGONNE LLC
Method for synthesizing trans-1,1,1,4,4,4-hexafluoro-2-butene
ActiveCN109553506AHigh catalytic activityThe reaction conditions are mild and controllablePreparation by hydrogen halide split-offPreparation by halogen replacementActive componentGas phase
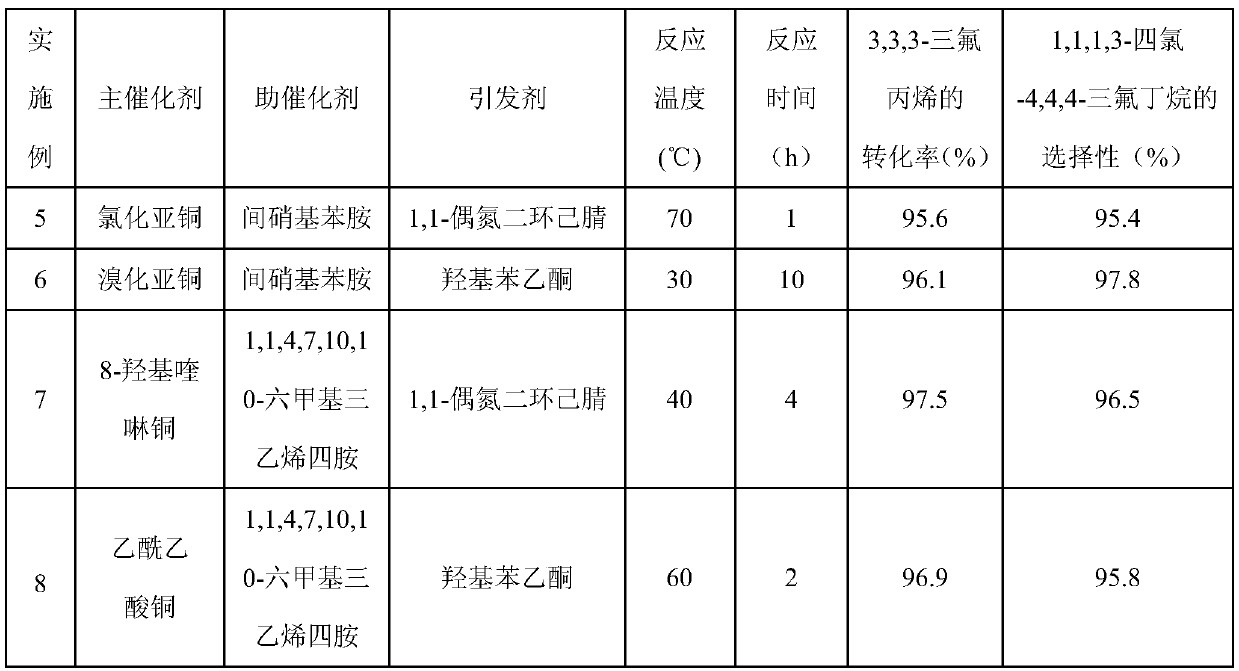
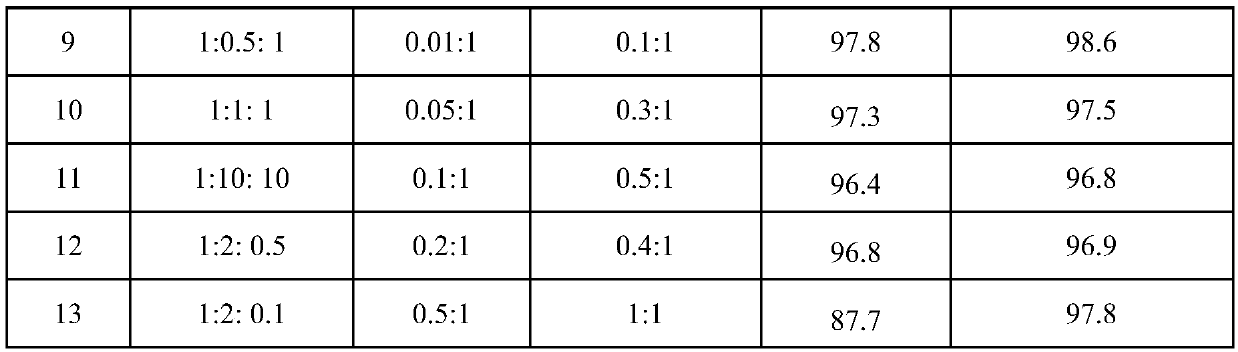
The invention discloses a method for synthesizing trans-1,1,1,4,4,4-hexafluoro-2-butene. The method comprises the following steps: (a) in the presence of a telomerization catalyst, performing a reaction on 3,3,3-trifluoropropene and carbon tetrachloride to synthesize 1,1,1,3-tetrachloro-4,4,4-trifluorobutane, wherein the reaction temperature is 30-70 DEG C, and the reaction time is 1-10 h; and (b)under the action of a fluorination catalyst, performing gas phase fluorination on the 1,1,1,3-tetrachloro-4,4,4-trifluorobutane and HF to synthesize the trans-1,1,1,4,4,4-hexafluoro-2-butene, whereinthe reaction temperature is 200-300 DEG C, a molar ratio of the HF to the 1,1,1,3-tetrachloro-4,4,4-trifluorobutane is (5-50):1, the telomerization catalyst is composed of a main catalyst, a cocatalyst and an initiator, the main catalyst is a monovalent or divalent copper salt, the cocatalyst is an organic amine, and the initiator is p-hydroxyacetophenone or 1,1-azobis(cyanocyclohexane); and thefluorination catalyst is a supported catalyst with a composition of Nx / MgF2, N is an active component and selected from one of Al, Cu, Zn and Co, x is a molar ratio of N to MgF2, and a value of x satisfies the relationship of 0.05 <= x <= 0.15. The method provided by the invention is mainly used for synthesizing the trans-1,1,1,4,4,4-hexafluoro-2-butene.
Owner:XIAN MODERN CHEM RES INST
Preparation method of highly dispersed nanocatalyst
ActiveCN108295848AGood dispersionNo aggregationCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsOxygen vacancyHydrogen atmosphere
The invention discloses a preparation method of a highly dispersed nanocatalyst. The method comprises the following steps: (1) respectively preparing a metal salt solution and a precipitant solution,continuously dropwise adding the metal salt solution into the precipitant solution under continuous stirring, continuing the stirring, performing a primary low-temperature hydrothermal reaction, filtering, washing and drying the obtained product, performing a secondary low-temperature hydrothermal reaction, and performing filtration and vacuum drying on the obtained reaction product to obtain a metal oxide carrier rich in oxygen vacancies; and (2) loading a noble metal on the metal oxide carrier by adopting an impregnation technology, and performing temperature-programmed reduction in a hydrogen atmosphere to obtain the highly dispersed nanocatalyst. The method has the advantages of low cost, rich and easily available raw materials, mild preparation conditions and strong universality; andthe highly dispersed nanocatalyst prepared by using the oxygen vacancy supported noble metal has the advantages of good catalytic activity, good product selectivity and great potential industrial catalytic application potential.
Owner:ZHEJIANG UNIV
Ruthenium catalysts
InactiveUS7618917B2High product selectivitySugar derivativesOrganic compound preparationHydrogenAlcohol sugars
Novel ruthenium catalysts can be obtained by:i) treating a support material based on amorphous silicon dioxide one or more times with a halogen-free aqueous solution of a low molecular weight ruthenium compound and subsequently drying the treated support material at below 200° C.,ii) reducing the solid obtained in i) by means of hydrogen at from 100 to 350° C.,where step ii) is carried out directly after step i). These catalysts can be used for the catalytic hydrogenation of monosaccharides and disaccharides to produce sugar alcohols, with the exception of sorbitol.
Owner:BASF AG
Preparation for composite titanium oxide catalyst of ester interchange synthetic phenyl ester oxalate
InactiveCN1583247AMild operating conditionsSimple processOrganic compound preparationCarboxylic acid esters preparationSilicon oxideHigh activity
A composite titanium oxide-silicon oxide as the catalyst for synthesizing phenyl oxalate by ester exchange, which is used to prepare diphenyl carbonate (DPC), is prepared through preparing the precursor solutions, mixing to obtain sol, depositing, drying and calcining. It has high activity.
Owner:TIANJIN UNIV
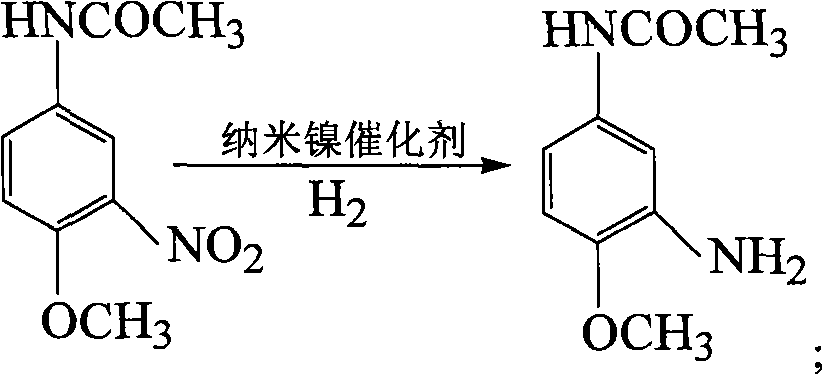
Method for synthesizing 3-amino-4-methacetin by catalytic hydrogenation
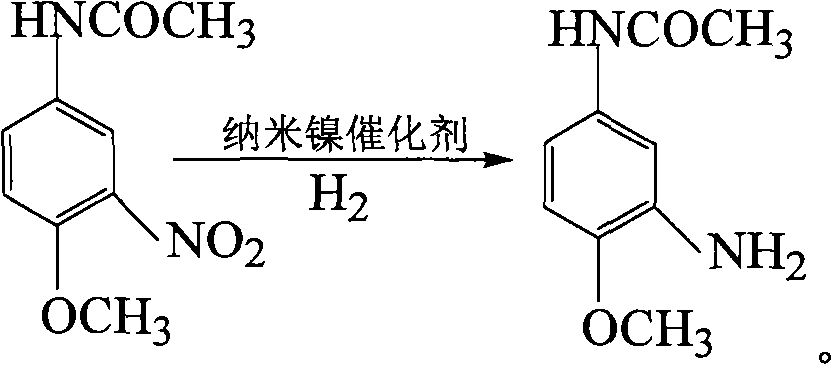
InactiveCN101565383AHigh activityHigh selectivityOrganic compound preparationCatalyst activation/preparationHydrazine compoundReaction temperature
The invention relates to a method for synthesizing 3-amino-4-methacetin by catalytic hydrogenation, which uses nano-nickle as a catalyst to catalyze 3-nitro-4-methacetin to obtain the 3-amino-4-methacetin through hydrogenation synthesis. The method comprises the following steps: respectively performing ultrasound processing on nickel oxalate and 1 to 50 weight percent of surfactant of the nickel oxalate and dissolving the nickel oxalate and the surfactant thereof into an ethanol solution; then adding the nickel oxalate and the surfactant thereof into a three-neck round-bottomed flask; mixing the nickel oxalate and the surfactant thereof under the stirring of a magnetic stirrer; adjusting the pH value to between 11 and 12; keeping reacting for 1 hour; under the mild stirring, adding a reducing agent of hydrazine hydrate to obtain a nano-nickle catalyst; dissolving the 3-nitro-4-methacetin into the ethanol solution, wherein the consumption of the nano-nickle catalyst is 1 to 20 percent of raw material weight; introducing nitrogen into a reaction kettle to displace air; and introducing the hydrogen into the reaction kettle at a reaction temperature of between 60 and 150 DEG C and at a reaction pressure of between 0.5 and 2.5 Mpa. After the reaction is finished, the conversion rate of the raw material of the 3-nitro-4-methacetin is between 85.5 and 100 percent, and the selectivity of the product of the 3-amino-4-methacetin is between 9.0 and 99.9 percent.
Owner:JIANGSU UNIV
Method for preparing iso-butyl aldehyde by using isobutene or tert-butyl alcohol as raw material
ActiveCN101260028AHigh selectivityHigh space-time yieldOrganic compound preparationCarbonyl compound preparationMethacroleinBismuth
The invention aims to provide a method of using isobutylene or tert-butyl alcohol as raw material to prepare isobutyraldehyde. The method prepares isobutyraldehyde through two steps as follows: (1) under the action of molybdenum-bismuth base composite oxide catalyst, isobutylene or tert-butyl alcohol selects oxidation to prepare methacrolein; (2) under the action of supported hydrogenation catalyst, methacrolein selects hydrogenation to prepare isobutyraldehyde. The raw material in the method is cheap and easy to get, which breaks the dependence of the prior isobutyraldehyde production on expensive C3 propylene raw material and opens up a brand new process route for preparing isobutyraldehyde through C4 raw material low in chemical utilization ratio. The method is high in the yield coefficient of isobutyraldehyde, simple in process, low in production cost and convenient for mass production.
Owner:SHANGHAI HUAYI NEW MATERIAL
Catalyst for benzene ring hydrogenation of terephthalic acid or dimethyl terephthalate
InactiveCN1915494AHigh product selectivityHigh reactivityCarboxylic preparation by ozone oxidationMetal/metal-oxides/metal-hydroxide catalystsDimethyl terephthalateActive component
A catalyst for hydrogenating the benzene ring of terephthalic acid or dimethy terephthalate is prepared from Ru as active component and Al2O3 as carrier through dissolving the soluble salt of Ru in deionized water,regulating pH=4-10, immersing Al2O3 in it for 0.5-5 hr, adding formaldehyde solution, reductive reaction, filtering and washing.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Method for synthesizing monatomic catalyst, monatomic catalyst and application
ActiveCN110961134AFix bugsSave raw materialsCatalyst activation/preparationElectrolytic organic productionNano catalystPtru catalyst
The invention belongs to the technical field of nano catalysts, and discloses a method for synthesizing a monatomic catalyst, the monatomic catalyst and an application. The method comprises the following steps: (1) under an acidic condition, polystyrene nanospheres are taken as a sacrificial template, and a cyanamide compound, furfural and a metal source fully react to obtain a high polymer, wherein the cyanamide compound is one or more of cyanamide, dicyandiamide or melamine, and the metal source is one or more of a Fe source, a Co source, a Ni source, a Pt source, an Au source and a Pd source; and (2) high-temperature pyrolysis is performed on the high polymer in the atmosphere of protective gas to obtain the monatomic catalyst. The method disclosed by the invention is simple, can be used for synthesizing various metal monatomic catalysts, is a universal method, can be used for synthesizing the monatomic catalyst on a large scale, and overcomes the defects of complex synthesis and difficulty in large-scale preparation of other existing technologies. The catalyst disclosed by the invention is excellent in performance, is used for electrocatalytic chemical reaction and is used as an electrocatalyst.
Owner:SOUTH CHINA UNIV OF TECH
Catalyst for preparing dimethyl ether as well as preparation method and application thereof
ActiveCN102600852APrevent sinteringOvercoming inactivationMetal/metal-oxides/metal-hydroxide catalystsEther preparation by compound dehydrationCompound aMixed oxide
The invention relates to a catalyst for preparing dimethyl ether. The catalyst is prepared by compounding a methanol synthesis catalyst precursor with a zirconia-aluminum oxide mixed oxide precursor, wherein the methanol synthesis catalyst precursor is prepared by adopting a coprecipitation method; and particularly, the catalyst is prepared by the steps of: adding the zirconia-aluminum oxide mixed oxide precursor to the methanol synthesis catalyst precursor, uniformly stirring and mixing, drying and calcining to prepare the bifunctional catalyst for synthesizing dimethyl ether, which is homogeneous in composition and structure. According to the invention, the preparing process of the catalyst is simple and good in repeatability; and when the catalyst is used for synthesizing dimethyl ether by co-hydrogenation of carbon monoxide carbon dioxide, the reaction conditions are mild, the catalytic activity is high and the stability is good, so that the problems of the traditional bifunctional catalyst system that the reaction activity is poor and two components interact are effectively solved.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Method for preparing bis((3, 4-epoxycyclohexyl) methyl) adipate by using microchannel reactor
ActiveCN109912544AImprove thermal stabilityImprove high temperature resistanceOrganic chemistryCyclohexeneDouble bond
The invention discloses a method for preparing bis((3, 4-epoxycyclohexyl) methyl) adipate by using a microchannel reactor. The method comprises the following steps: using 3-cyclohexene-1-methanol andhexanedioyl chloride as raw materials for performing esterification reaction in a first microreactor to prepare an intermediate bis((3-cyclohexenyl)methyl) adipate; using peroxyacetic acid as an oxidant; performing selective epoxidation on carbon-carbon double bonds of cyclohexenyl on the intermediate bis((3-cyclohexenyl)methyl) adipate in a second microreactor to prepare bis((3, 4-epoxycyclohexyl) methyl) adipate. The method provided by the invention adopts an in-situ method, performs epoxidation reaction of olefin while preparing peroxyacetic acid, and has the advantages of easily operable process, low esterification reaction temperature, few side reactions, simple post-treatment, high efficiency, high reaction yield, high product purity, obvious improvement in conversion rate of the intermediate product and selectivity of the product, simple product separation process and operation, and suitability for a continuous production process.
Owner:JIANGSU TETRA NEW MATERIAL TECH
Method for preparing (methyl)acrylic acid-3,4-epoxycyclohexyl methyl ester by microchannel reactor
ActiveCN108299343ARaw materials are cheap and easy to getEasy to operateOrganic chemistryCyclohexeneMethyl group
The invention discloses a method for preparing (methyl) acrylic acid-3,4-epoxycyclohexyl methyl ester by a microchannel reactor. The method is characterized in that firstly, 3-cyclohexene-1-methanol and (methyl)acryloyl chloride are used as raw materials; esterification reaction is performed in the microchannel reactor to prepare an intermediate of (methyl)acrylic acid-3-cyclohexenyl methyl ester;then, peroxyacetic acid is used as an oxidizing agent; chlorohydrocarbon is used as a reaction medium; selective epoxidation reaction is performed in the microchannel reactor to prepare a target product. The esterification reaction temperature is low; the side reactions are few; the efficiency is high; the aftertreatment is simple; an in-situ method is used; peroxyacetic acid is prepared; meanwhile, olefin epoxidation reaction is performed; the technological process is easy to operate; the reaction yield is high; the product purity is high; the operation of the product separation process is simple; the method is suitable for continuous production; when a curing resin composition prepared by the product is used, a good cured matter with high sealing performance and chemical resistant performance on a base material can be obtained, and can be suitable for being used in the field of electronic materials.
Owner:JIANGSU TETRA NEW MATERIAL TECH
Method for preparing loaded zeolite-like imidazole framework material and application of loaded zeolite-like imidazole framework material in cyclohexane oxidation reaction
InactiveCN106582859AImprove conversion rateHigh product selectivityPreparation by oxidation reactionsOrganic compound preparationSolventChemistry
The invention discloses a method for preparing a loaded zeolite-like imidazole framework material and application of the loaded zeolite-lie imidazole framework material in cyclohexane oxidation reaction. The method comprises the following steps: firstly, preparing a zeolite-like imidazole framework material by adopting a solvothermal method; then, loading the zeolite-like imidazole framework material; and applying the zeolite-like imidazole framework material to the cyclohexane oxidation reaction. By adopting the loaded zeolite-like imidazole framework material, the raw materials are low in prices, and the catalyst has a short preparation cycle and simple process, so that the cost is remarkably reduced; the loaded zeolite-like imidazole framework material is used in the cyclohexane oxidation reaction, a relatively cyclohexane conversion rate and product selectivity can be represented, and the catalyst has excellent reproducibility and can be easily recycled, so that the loaded zeolite-like imidazole framework material has excellent industrial application prospect.
Owner:XIANGTAN UNIV
Method for preparing diphenyl ketone employing biomimetic catalysis of diphenylmethane and oxygen oxidation
ActiveCN104478677AImprove efficiencyHigh product selectivityOrganic compound preparationCarbonyl compound preparationDiphenyl ketoneReactions stress
The invention discloses a method for preparing diphenyl ketone employing biomimetic catalysis of diphenylmethane and oxygen oxidation. The method comprises the following steps: with diphenylmethane as a raw material and oxygen as a catalyst, adding a hydrogen carrier; with a metal porphyrin compound as a catalyst, carrying out catalytic reaction under the conditions that the reaction temperature is controlled to 50-150 DEG C and the reaction pressure is controlled to 0.2-2.0MPa, so as to obtain diphenyl ketone. The method has the advantages of mild reaction condition, and good catalysis effect; and the product is high in selectivity and simple in process.
Owner:SUN YAT SEN UNIV
Aromatization Catalyst Preparation with Alkali Metal Present During a Washing Step
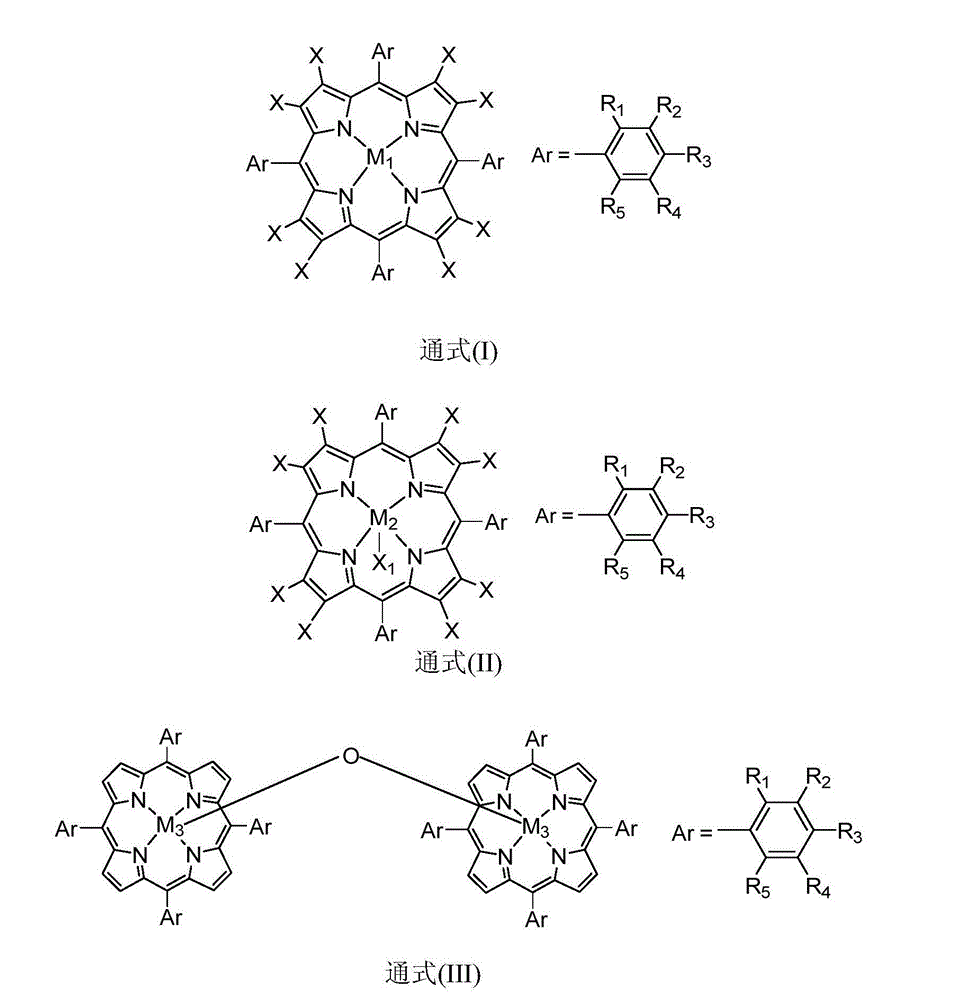
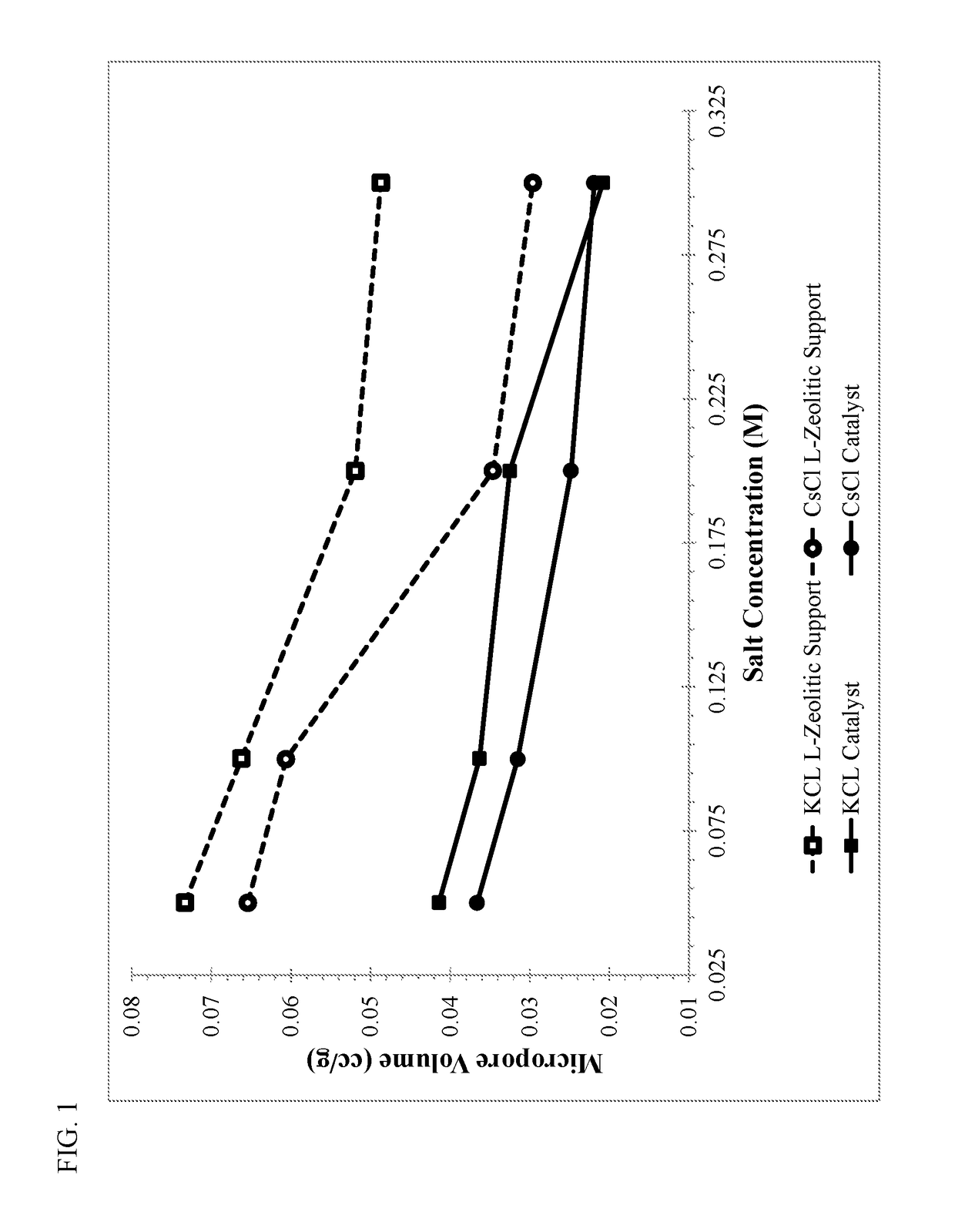
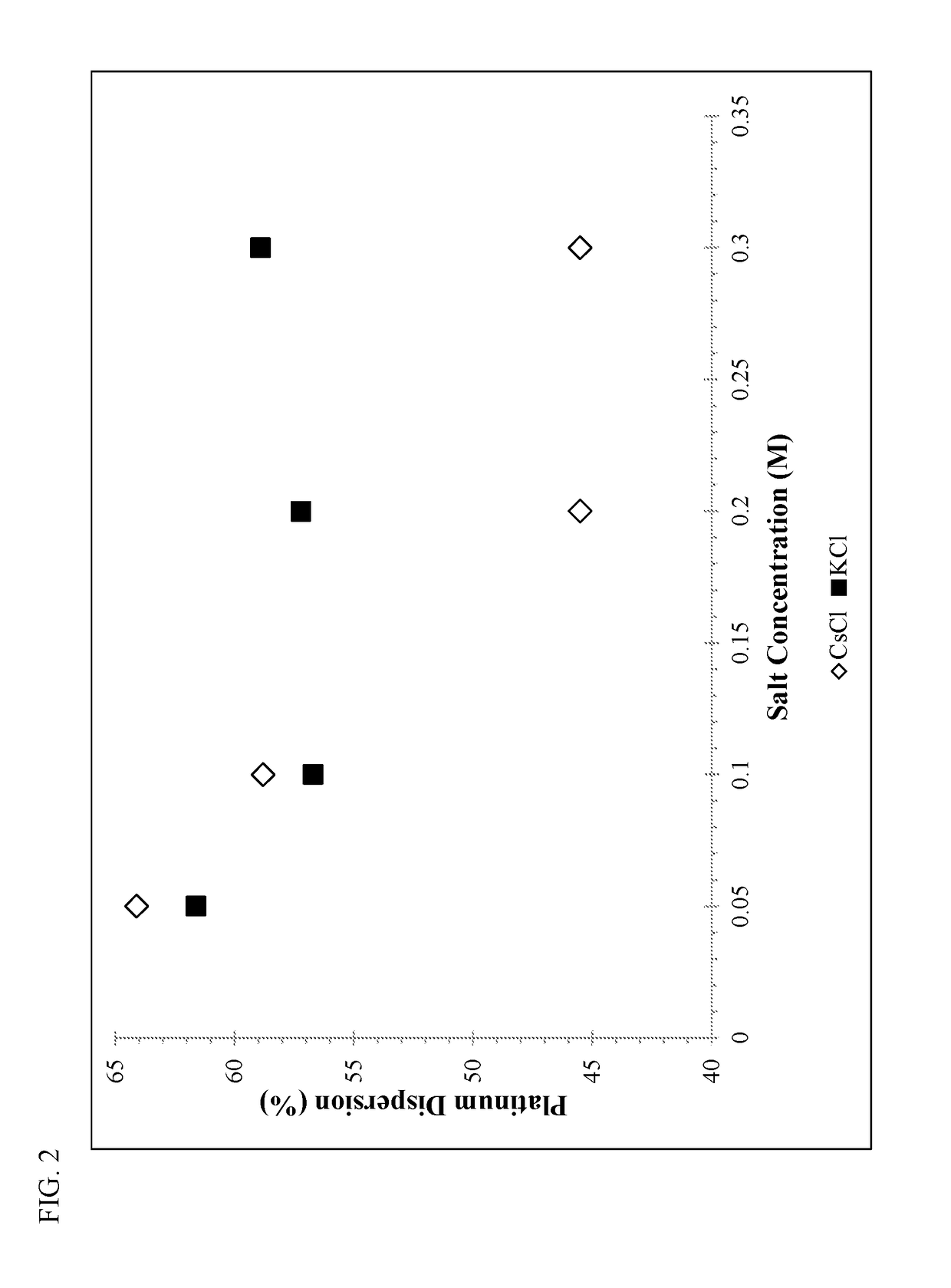
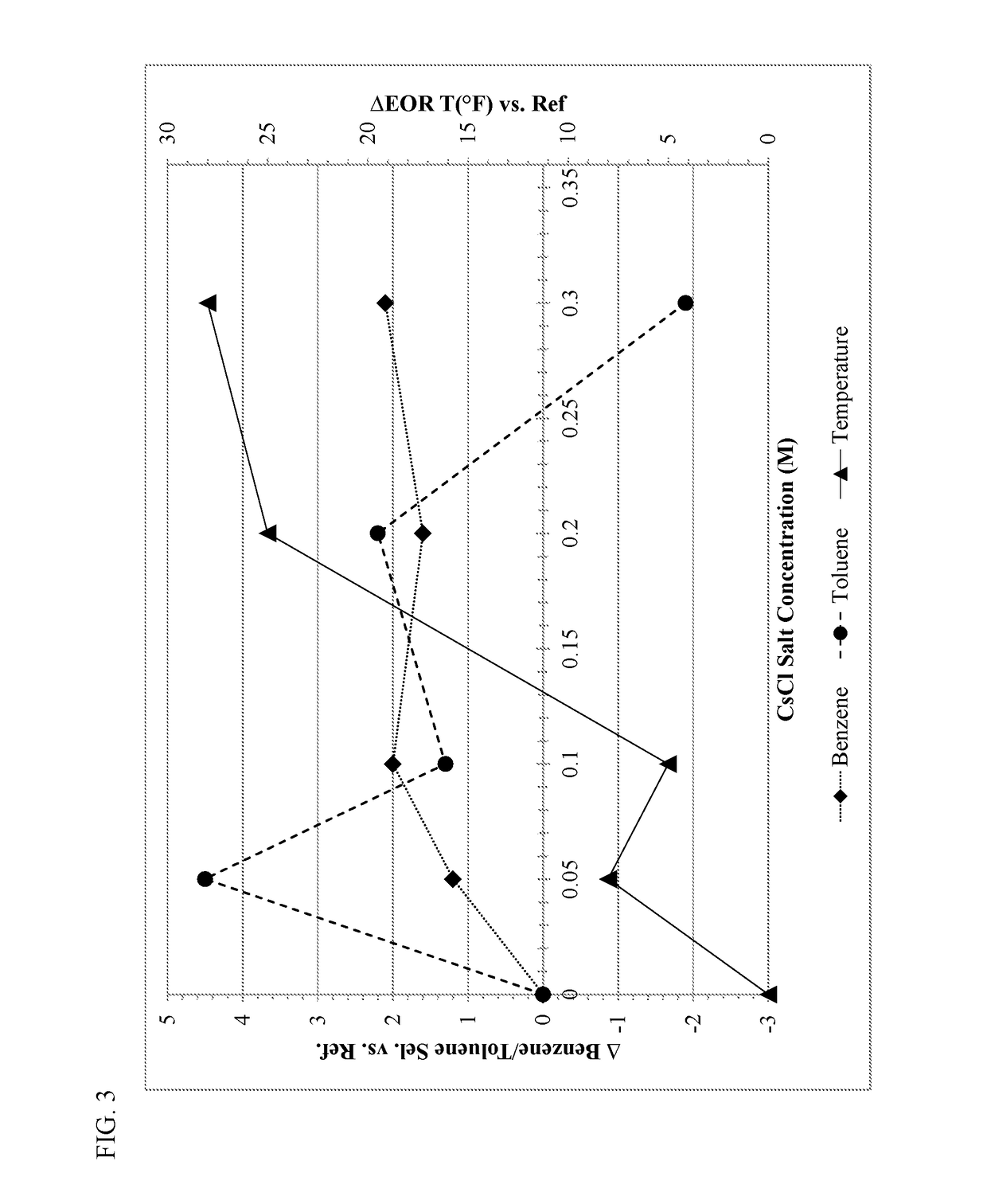
ActiveUS20180169638A1High product selectivitySmall surface areaMolecular sieve catalystsRefining to change hydrocarbon structural skeletonAromatizationPotassium
Methods for producing supported catalysts containing a transition metal and a bound zeolite base are disclosed. These methods employ a step of washing the bound zeolite base in the presence of an alkali metal, prior to impregnating the bound zeolitic support with the transition metal. Alkali metals such as potassium and cesium may be used.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
MWW type molecular sieve carrier catalyst and preparation method and application thereof
InactiveCN103028430AEffectively regulates acidityLarge specific surface areaCatalyst carriersMolecular sieve catalystsCooking & bakingSide chain
The invention discloses an MWW type molecular sieve carrier catalyst and a preparation method and an application thereof, namely a catalyst for the alkylation reaction of toluene and carbinol side chains and a preparation method and an application of the same. The MWW type molecular sieve carrier catalyst comprises a carrier body, and a boron element and alkali-earth metal which are loaded on the carrier body. The preparation method comprises the following steps of: (a) exchanging ammonium salt, and baking into an H-type MWW type molecular sieve; (b) exchanging alkaline-earth metal salt by exchange liquid; and (c) baking, and loading on the molecular sieve by a boric acid solution impregnation method to obtain the MWW type molecular sieve carrier catalyst. The catalyst prepared by the method has excellent hydrothermal stability and special acid site distribution, the alkalinity of the catalyst can be enhanced, and the catalytic activity and selectivity can be improved. The MWW type molecular sieve is modified by alkaline-earth metal oxide through the impregnation method, the acid-alkali property of the MWW type molecular sieve can be modulated, and the novel catalyst having acid-alkali double functions is prepared.
Owner:NANJING UNIV OF TECH
Method for synthesizing hexamethylenediamine key intermediate
ActiveCN111978207AReduce caprolactam polymerizationReduce surface cokingOrganic compound preparationCarboxylic acid amides preparationLactamMaterials science
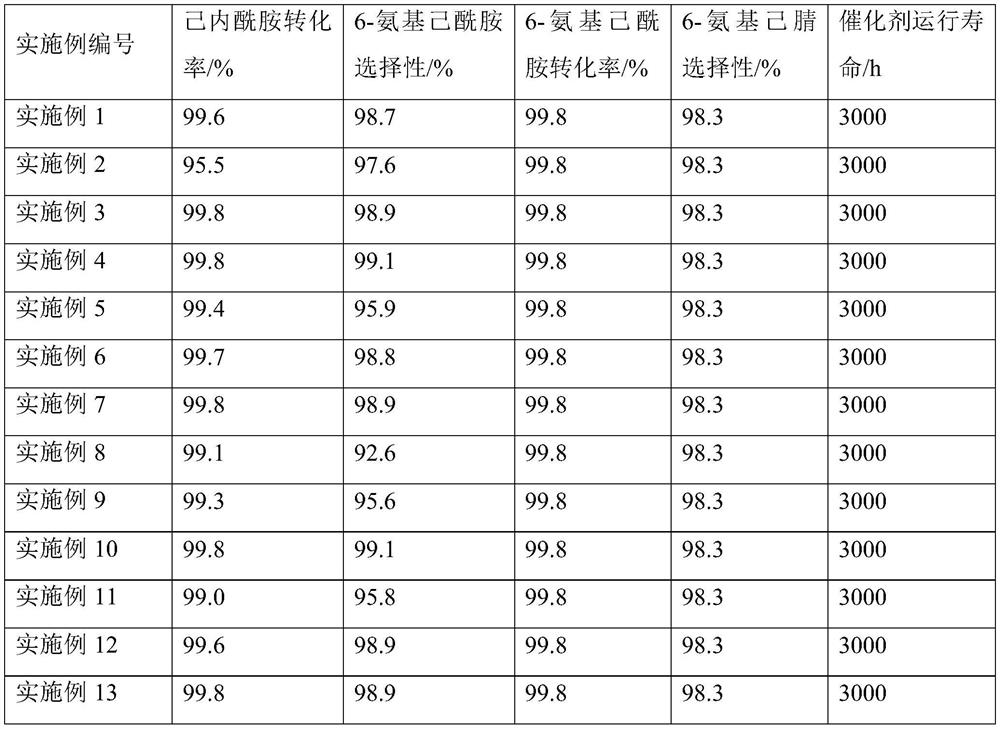
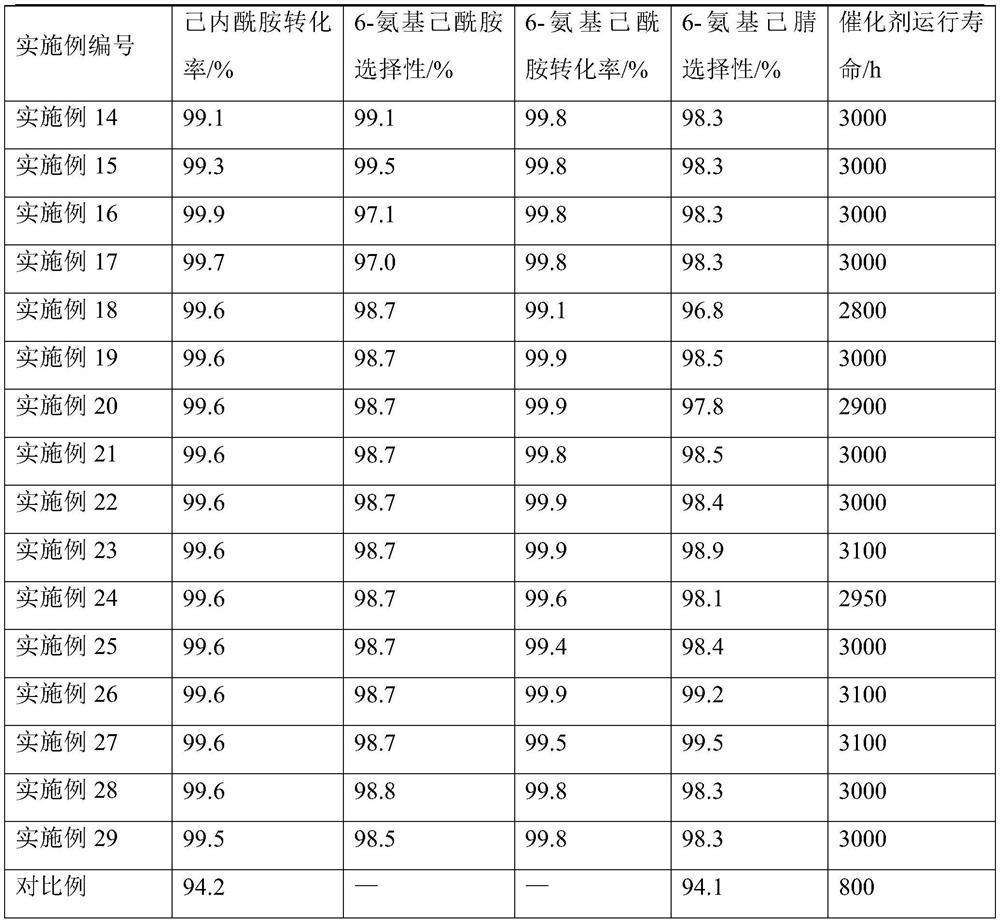
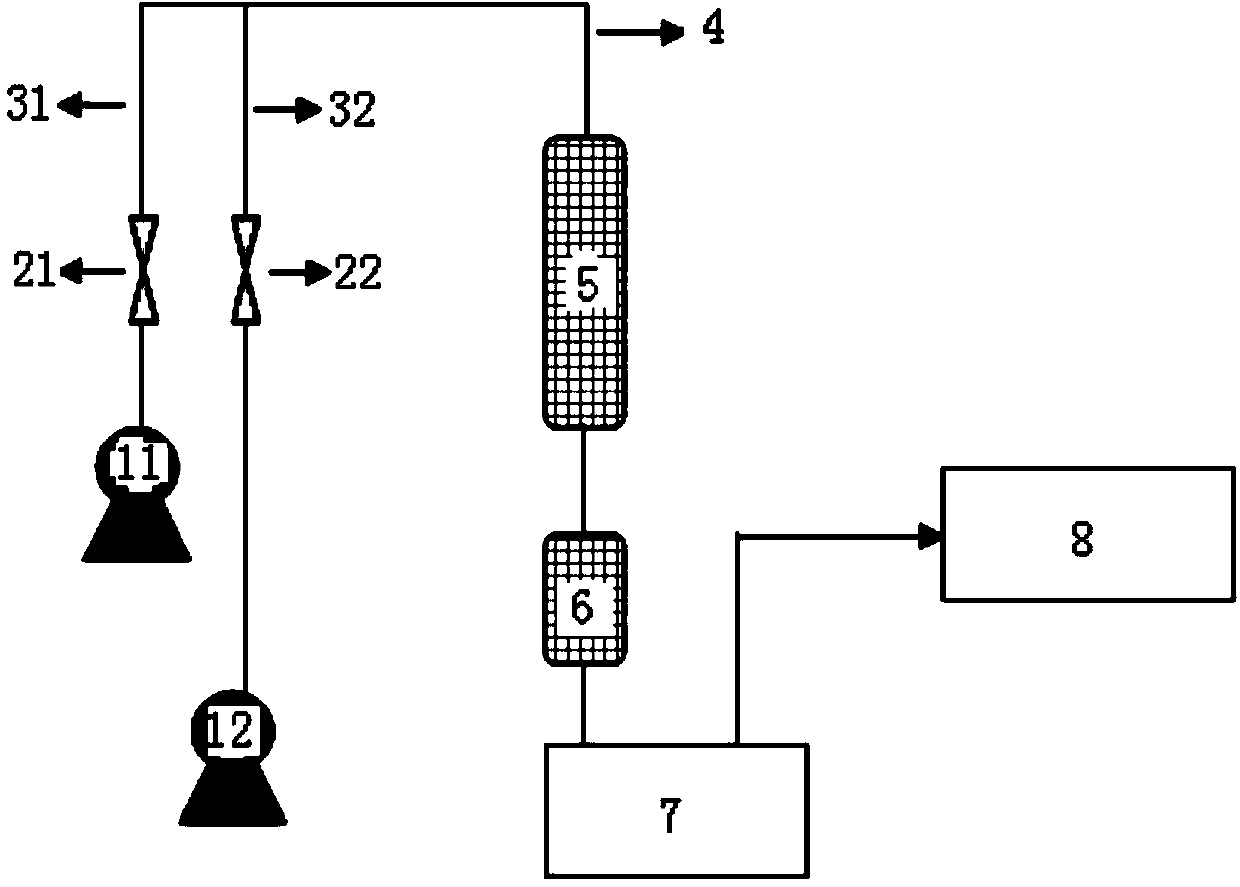
The invention relates to a method for synthesizing a hexamethylenediamine key intermediate, and is particularly suitable for synthesizing a hexamethylenediamine key intermediate namely 6-aminocapronitrile. The method comprises the following steps of preheating caprolactam and ammonia water, respectively introducing the caprolactam and the ammonia water into a micro-channel reactor, and performingreaction; separating the material obtained after the reaction to obtain 6-aminocaproamide; and preheating and gasifying the 6-aminocaproamide obtained by separation, introducing the gasified 6-aminocaproamide into a reactor loaded with a catalyst, and performing a reaction to obtain the 6-aminocapronitrile. An existing process route is broken through, the hexamethylenediamine key intermediate namely the 6-aminocapronitrile is synthesized more efficiently by an innovative method, a 6-aminocaproamide intermediate is generated through caprolactam ring-opening hydrolysis, and then dehydrating is performed to generate the 6-aminocapronitrile, so that caprolactam polymerization is effectively reduced, catalyst surface coking is reduced, the utilization rate of raw materials is increased, the selectivity of products is improved, the service life of the catalyst is prolonged, the process flow is simple, the production cost is low, and the method is suitable for industrial production.
Owner:JIANGSU YANGNONG CHEM GROUP +3
Heteropolyacid catalyst and preparation method thereof
ActiveCN104801342AExtended service lifeHigh catalytic activityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSaline solutionsAlum
The invention provides a heteropolyacid catalyst and a preparation method thereof. The chemical formula of the catalyst is as follows: PaMo12VbAcBdCeDfOg. Solid heat-conduction powder is added to an aqueous solution containing phosphorus, molybdenum and alum, so that molybdovanadophosphoric acid generated in a reaction is attached to the surface of the solid heat-conduction powder, then other saline solutions are added, and heteropolyacid deposition layers are uniformly supported on surfaces of solids. The catalyst with good product selectivity, long service life and high mechanical strength is obtained through drying, roasting, forming and other procedures. The catalyst obtained with the preparation method can be applied to selective oxidation preparation of methacrylic acid through methylacrolein.
Owner:YANTAI UNIV
Solid acid catalyst and preparation method thereof, and preparation method of diolefin compound
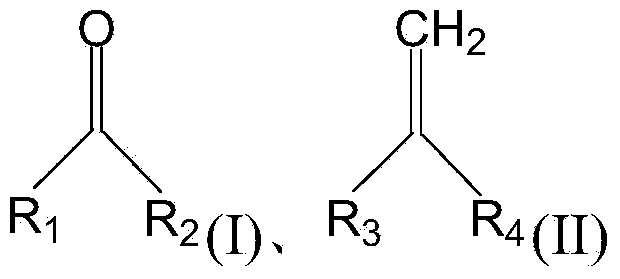
ActiveCN103721729AHigh product selectivityLess side effectsCatalyst carriersHydrocarbon by hydrocarbon and non-hydrocarbon condensationCarbonyl groupSide reaction
The invention provides a solid acid catalyst which comprises the following components: metallic oxide, auxiliaries and a carrier, wherein the auxiliaries comprise boric oxide and phosphorus oxide. The solid acid catalyst provided by the invention can catalyze the condensation reaction of a carbonyl compound with a structure shown in the formula (I) and a monoolefin compound with a structure shown in the formula (II), so as to prepare a diene compound. The solid acid catalyst provided by the invention has relatively high product selectivity, the side reaction during the condensation reaction is reduced and the productivity of the diene compound is improved; the solid acid catalyst provided by the invention has high catalytic activity and a relatively high conversion rate; moreover, the solid acid catalyst provided by the invention has low toxicity, and is long in service life and good in regenerability, thereby being applicable to industrial production.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Catalyst for preparing acrylic acid and butyl ester using lactic acid method and preparation method thereof
ActiveCN101417232AImprove crystal structureHigh strengthPhysical/chemical process catalystsOrganic compound preparationSulphate IonBUTYL LACTATE
The invention discloses a catalyst used for preparing acrylic acid and butyl ester by the dehydration of butyl lactate and a preparation method thereof. The catalyst is a composite catalyst, comprising a main catalyst A and auxiliary catalysts B, C, D and E, wherein, A is calcium sulphate, B is sulphuric acid, C is phosphate, D is alkaline matter and E is promoter. The preparation method comprises the steps as follows: five compositions A, B, C, D and E are sufficiently mixed, baked for 2-10 hours at the temperature of 200-800DEG C and formed for standby. The catalyst has high catalytic activation; under the catalysis of the catalyst, the molar yield reaches 75.1 percent and the molar selectivity of the acrylic acid and butyl ester reaches 71.5 percent. The preparation method has the advantages that the crystal structure of the main catalyst can be improved so as to be beneficial for carrying out the reaction; and the introduction of different auxiliary catalysts and quantities is helpful to improve the conversion ratio of the raw material and the product selectivity and is beneficial for improving the strength of the catalyst.
Owner:溧阳常大技术转移中心有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com