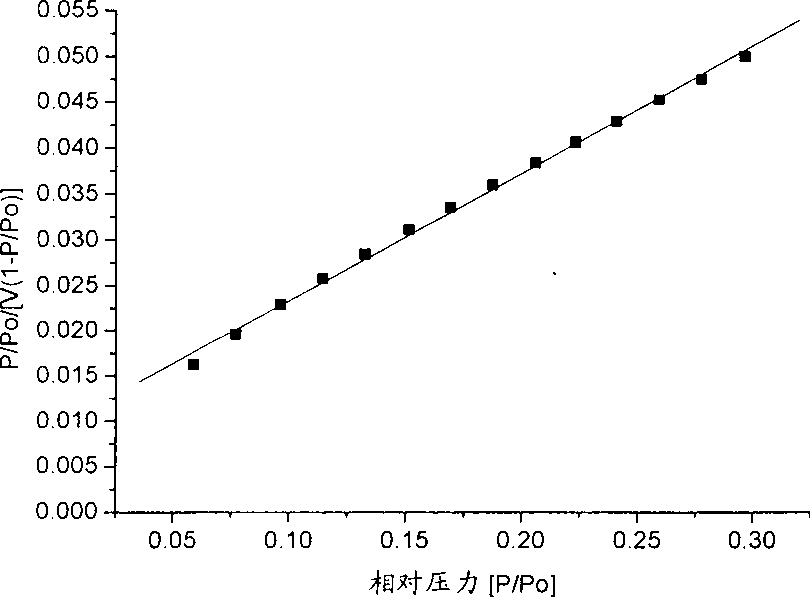
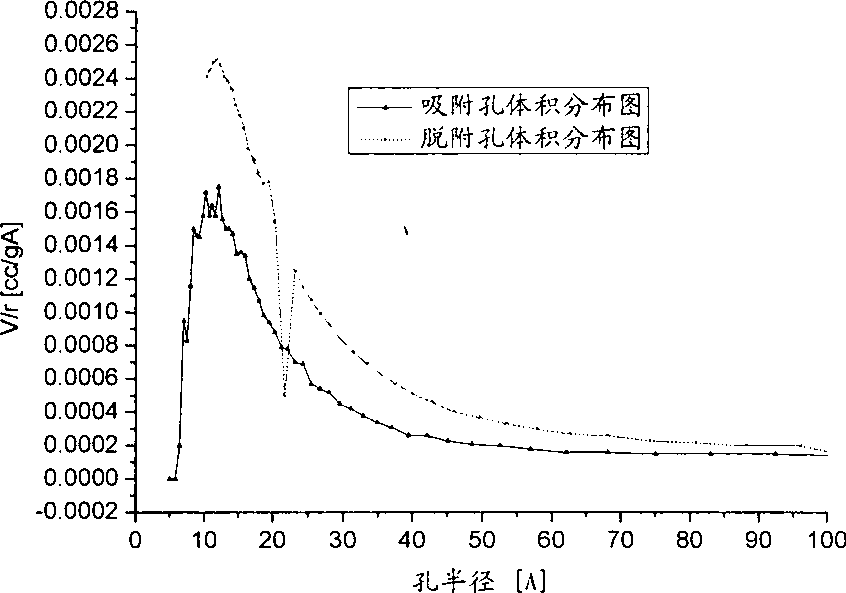
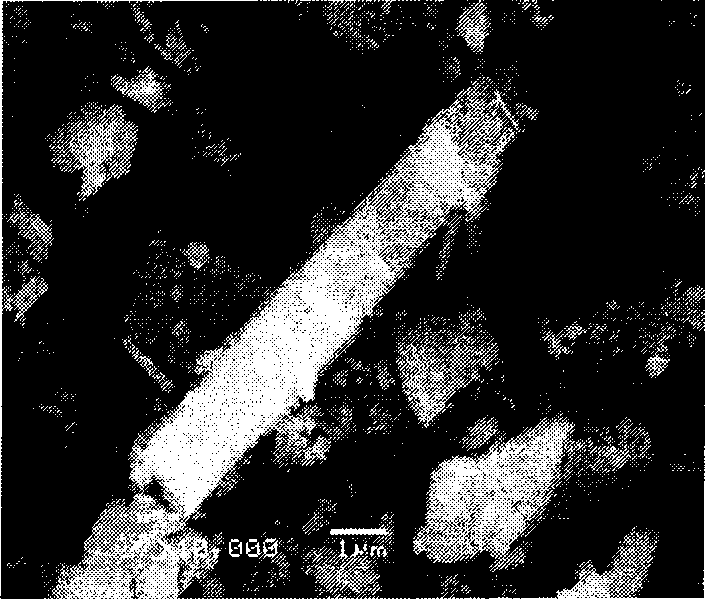Catalyst for preparing acrylic acid and butyl ester using lactic acid method and preparation method thereof
A catalyst, acrylic technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1: Weigh a quantitative amount of CaSO 4 , CuSO 4 , NaH 2 PO 4 , La 2 O 3 , After being fully grinded in an agate mortar and transferred to a beaker, the molar ratio of raw materials: CaSO 4 :CuSO 4 :NaH 2 PO 4 :La 2 O 3 =50:1:1:3;
[0029] Step 2: Put H 3 PO 4 Dissolved in quantitative deionized water (the amount of deionized water is CaSO 4 , CuSO 4 , NaH 2 PO 4 , La 2 O 3 The total mass of ), add it to the beaker in step 1, mix and stir thoroughly, the molar ratio of raw materials: CaSO 4 :H 3 PO 4 =50:2;
[0030] Step 3: Dry the material in step 2 in an oven at 110°C until there is no free water, grind and pulverize, sieving with a standard sieve of 18 mesh and 35 mesh to obtain a catalyst of 18 to 35 mesh;
[0031] Step 4: Roast the catalyst prepared in step 3 at 450°C for 4 hours, and place it in a desiccator for use after cooling;
[0032] Step 5: The catalyst prepared in Step 4 is used in the reaction of butyl lactate dehydration to prepare acrylic acid and its...
Embodiment 2
[0034] Step 1: Weigh a quantitative amount of CaSO 4 , CuSO 4 , NaH 2 PO 4 , La 2 O 3 , After being fully grinded in an agate mortar and transferred to a beaker, the molar ratio of raw materials: CaSO 4 :CuSO 4 :NaH 2 PO 4 :La 2 O3 =50:3:1:3;
[0035] Step 2 to Step 4: Same as Step 2 to Step 4 in Example 1.
[0036] Step 5: The catalyst prepared in Step 4 is used in the reaction of butyl lactate dehydration to prepare acrylic acid and its butyl ester, the specific reaction conditions: temperature 400℃, pressure at normal pressure, space velocity 1.8h -1 , Adopt nitrogen protection, the feed volume ratio of nitrogen and butyl lactate is: V (nitrogen): V (raw material) = 40:1, the experimental result: the molar conversion rate of butyl lactate reaches 75.7%, acrylic acid and its butyl ester The molar selectivity is 70.5%.
Embodiment 3
[0038] Step 1: Weigh a quantitative amount of CaSO 4 , MgSO 4 , NaH 2 PO 4 , La 2 O 3 , After being fully grinded in an agate mortar and transferred to a beaker, the molar ratio of raw materials: CaSO 4 :MgSO 4 :NaH 2 PO 4 :La 2 O 3 =50:1:1:3;
[0039] Step 2 to Step 4: Same as Steps 2 to 4 in Example 1.
[0040] Step 5: The catalyst prepared in step 4 is used in the dehydration reaction of butyl lactate to prepare acrylic acid and its butyl ester. The specific reaction conditions are: temperature 400℃, pressure is normal pressure, and space velocity is 1.8h -1 , Using nitrogen protection, the feed volume ratio of nitrogen and butyl lactate is: V (nitrogen): V (raw material) = 40:1, experimental results: the molar conversion rate of butyl lactate reaches 69.1%, acrylic acid and its butyl ester The molar selectivity reached 65.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com