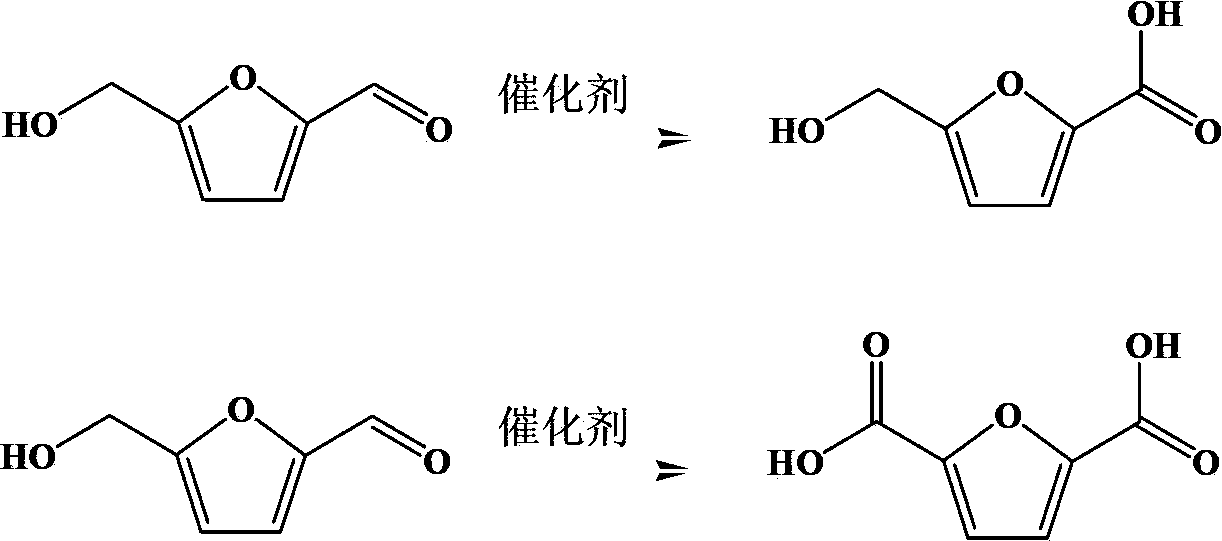
Preparation method of 5-hydroxymethyl furoic acid and 2,5-furandicarboxylic acid
A technology of hydroxymethylfuroic acid and furandicarboxylic acid, which is applied in chemical instruments and methods, molecular sieve catalysts, physical/chemical process catalysts, etc. Inactivation and other problems, to achieve the effect of high reaction efficiency and product selectivity, mild reaction conditions and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
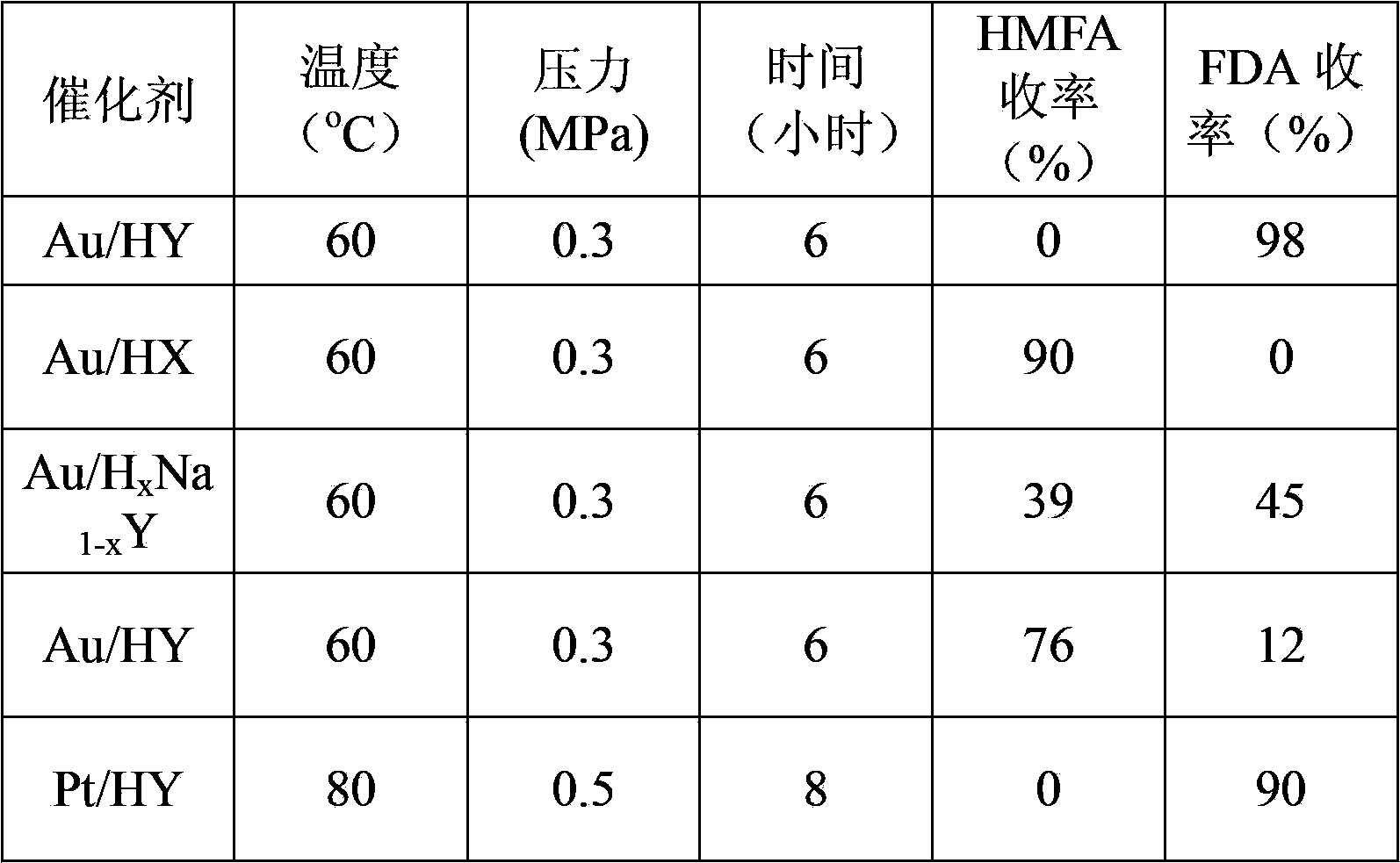
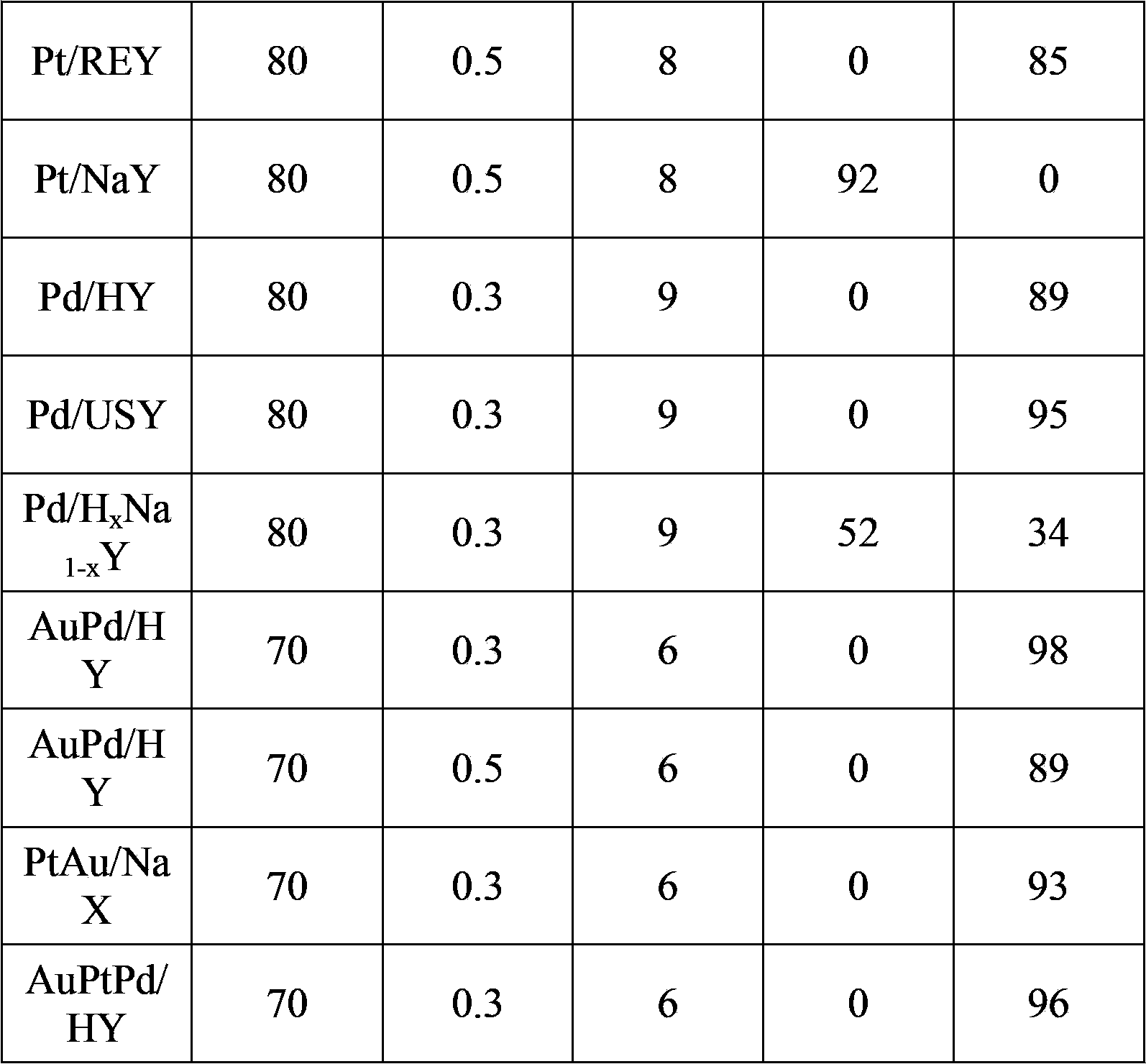
[0033] Take 1.73 g HAuCl 4 Add the solution (1wt%) to 50 grams of deionized water, heat to 60°C, add 0.3 grams of HY and stir, add sodium citrate solution for reduction and stir for 2 hours, wash the catalyst to PH = 7, and bake it under a hydrogen atmosphere at 100°C After drying for 5 hours, the catalyst Au / HY was finally obtained.
[0034] Take 0.317 g of HMF, 2.8 g of KOH (20%) and 3 ml of water into the reactor. The reaction vessel is a high-pressure reactor with 10 ml of polytetrafluoroethylene lining inside. Take 0.30 g of Au / HY (Au2wt%) as Catalyst, after temperature program is raised to 60 ℃, be filled with 0.3MPa oxygen, react for 6 hours, constantly supplement oxygen in the reaction process, guarantee that reaction is carried out under constant temperature and pressure. After the reaction product was centrifuged, the supernatant was removed and analyzed by HPLC. After testing, the conversion rate of the raw material HMF was 100%, and the FDA yield was 98%. The rea...
Embodiment 2
[0036] Take 1.73 g HAuCl 4 Add the solution (1wt%) to 50 grams of deionized water, heat to 60°C, add 0.3 grams of HX and stir, add sodium borohydride solution for reduction and stir for 2 hours, wash the catalyst to PH=7, and bake in a hydrogen atmosphere at 100°C After drying for 5 hours, the catalyst Au / HX was finally obtained.
[0037] Take 0.317 g of HMF, 2 g of NaOH (20%) and 3 ml of water into the reactor. The reaction vessel is a high-pressure reactor with 10 ml of Teflon lining inside. Catalyst, after temperature program is raised to 60 ℃, be filled with 0.3MPa oxygen, react for 6 hours, constantly supplement oxygen in the reaction process, guarantee that reaction is carried out under constant temperature and pressure. After the reaction product was centrifuged, the supernatant was removed and analyzed by HPLC. After testing, the conversion rate of raw material HMF was 100%, and the yield of HMFA was 90%. The reaction results are shown in Table 1.
Embodiment 3
[0039] Dissolve 1 g of NaY in 50 g of deionized water, heat up to 60°C, add 0.1 g of NH 4 CL was mixed and stirred for 2 hours, washed and centrifuged until the solution pH=7, and dried at 200°C for 4 hours to obtain the exchanged H x Na 1-x Y. Take 1.73 g HAuCl 4 The solution (1wt%) was added to 50 g of deionized water, heated to 60°C, and 0.3 g of H x Na 1-x Stir with Y, add sodium citrate solution to reduce and stir for 2 hours, wash the catalyst to PH=7, and dry it for 5 hours under a hydrogen atmosphere at 100 ° C to finally obtain the catalyst Au / H x Na 1-x Y.
[0040] Take 0.317g of HMF, 2g of NaOH (20%), and 3ml of water into the reactor. x Na 1-x Y (Au2wt%) is the catalyst. After the temperature is programmed to 60°C, 0.3MPa oxygen is charged, and the reaction is carried out for 6 hours. During the reaction, oxygen is continuously replenished to ensure that the reaction is carried out at constant temperature and pressure. After the reaction product was centri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com