Method for synthesizing trans-1,1,1,4,4,4-hexafluoro-2-butene
A technology of butene and trifluorobutane, applied in the field of synthesizing trans-1,1,1,4,4,4-hexafluoro-2-butene, which can solve the problem of high reaction temperature and no stability evaluation and other problems to achieve the effect of high catalytic activity and mild and controllable reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
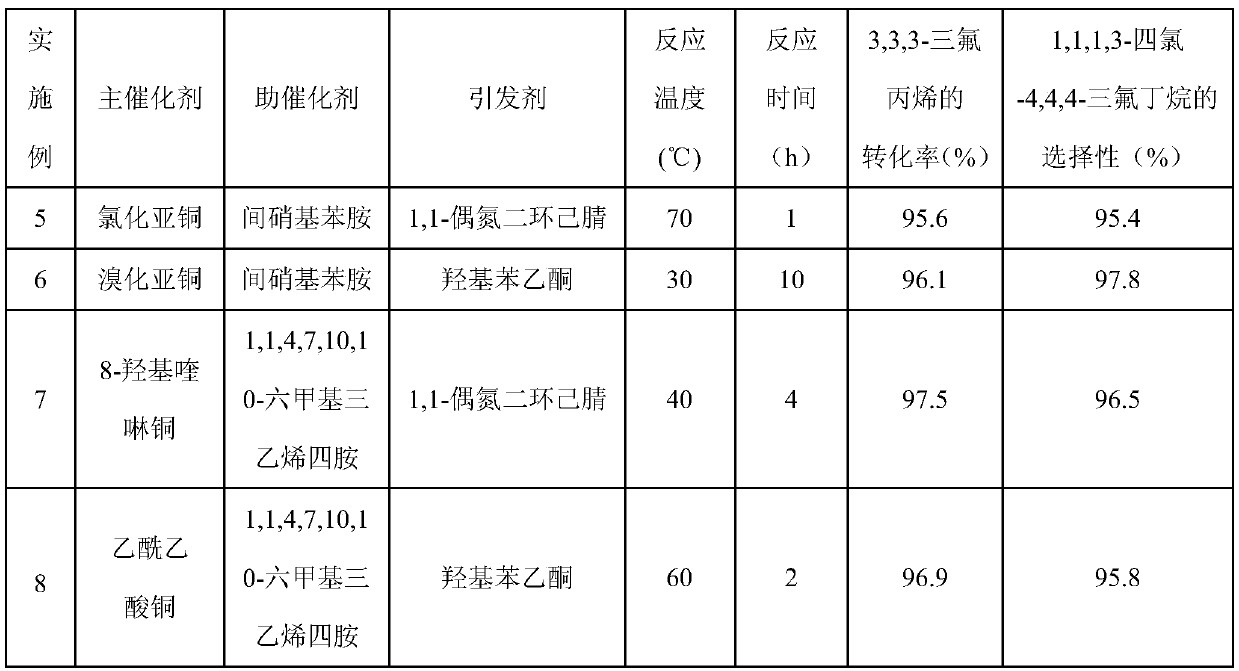
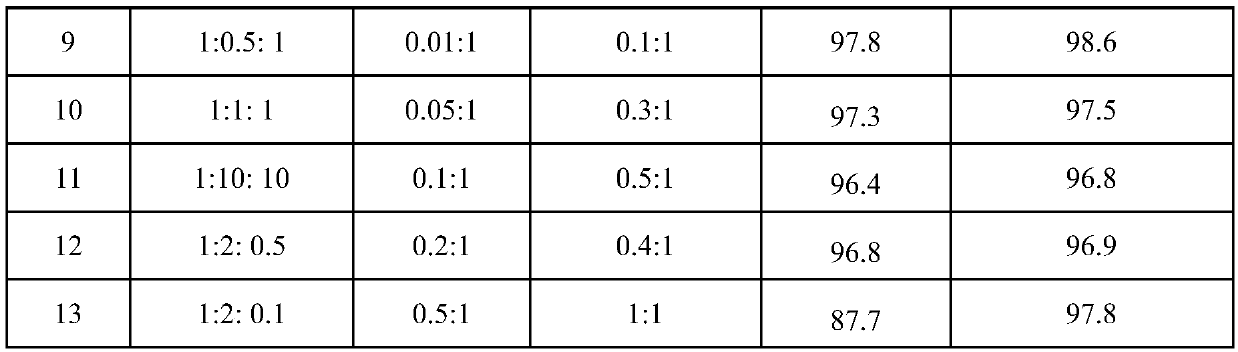
[0029] With main catalyst copper bromide 11.17g (0.05mol), procatalyst p-fluoroaniline 5.56g (0.05mol), initiator azobisisobutyronitrile 6.81g (0.05mol) and CCl 4 770g (5mol) was added to a 1L stainless autoclave. Seal the autoclave, start stirring, and replace the air in the autoclave with nitrogen for three times. Then, 240.13 g of 3,3,3-trifluoropropene was added at one time, the temperature of the reactor was heated to 60° C., and the reaction was completed after 4 hours. After the autoclave was lowered to room temperature, the autoclave was unloaded and the material was taken out. A liquid sample was taken with a pipette and analyzed by chromatography. The conversion rate of 3,3,3-trifluoropropene was 97.5%, 1,1,1,3-tetrachloro-4,4,4-trifluorobutane The selectivity of 1,1,1,3-tetrachloro-4,4,4-trifluorobutane was 96.6%, and the yield of 1,1,1,3-tetrachloro-4,4,4-trifluorobutane was 94.2%.
Embodiment 2
[0031] Add 0.08mol of MgF to the beaker 2 Support, impregnated with AlCl by isometric impregnation 3 ·6H 2 O, AlCl 3 ·6H 2 O and MgF 2 The molar ratio is 0.05, dried in an oven at 100°C for 8h, and calcined in a muffle furnace at 450°C for 1h to make a catalyst 5Al / MgF 2 .
[0032] Measure 5mL of the catalyst prepared by the above steps and put it into the reaction tube. The time is 6s, the molar ratio of HF and 1,1,1,3-tetrachloro-4,4,4-trifluorobutane is 10:1, and the operation is 24h. After the reaction product is washed with water to remove acid and dried, the gas chromatography Analysis showed that the conversion rate of 1,1,1,3-tetrachloro-4,4,4-trifluorobutane was 100%, and the selectivity of E-HFO-1336mzz was 97.9%.
Embodiment 3
[0034] The operation process of embodiment 3 is similar to embodiment 1, and difference is that main catalyst is cupric chloride. The product was analyzed by chromatographic method, the conversion rate of 3,3,3-trifluoropropene was 96.5%, the selectivity of 1,1,1,3-tetrachloro-4,4,4-trifluorobutane was 95.8%, The selective yield of 1,1,1,3-tetrachloro-4,4,4-trifluorobutane was 92.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com