Tenofovir preparation method suitable for industrialized production
A tenofovir product technology, applied in the field of drug synthesis, can solve the problems affecting the yield of industrialized large-scale production process, hindering the crystallization of tenofovir, and demanding filtration equipment, etc., to achieve convenient industrial production, good reproducibility, easy-to-handle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
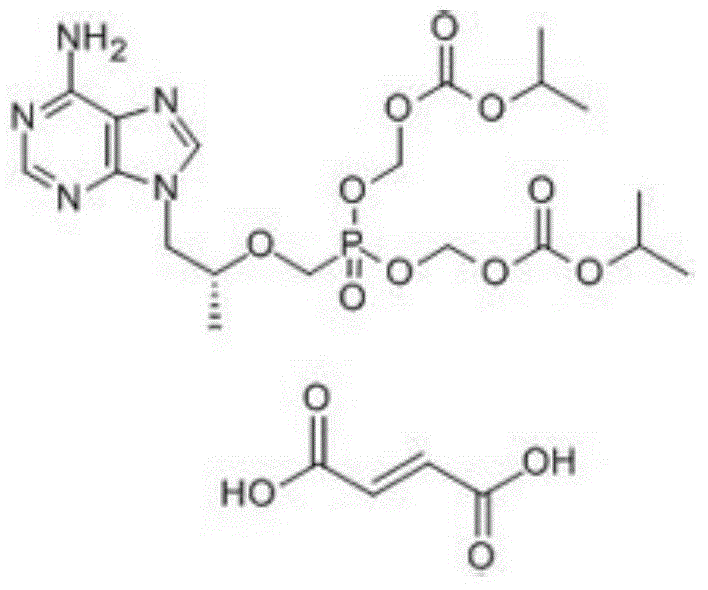
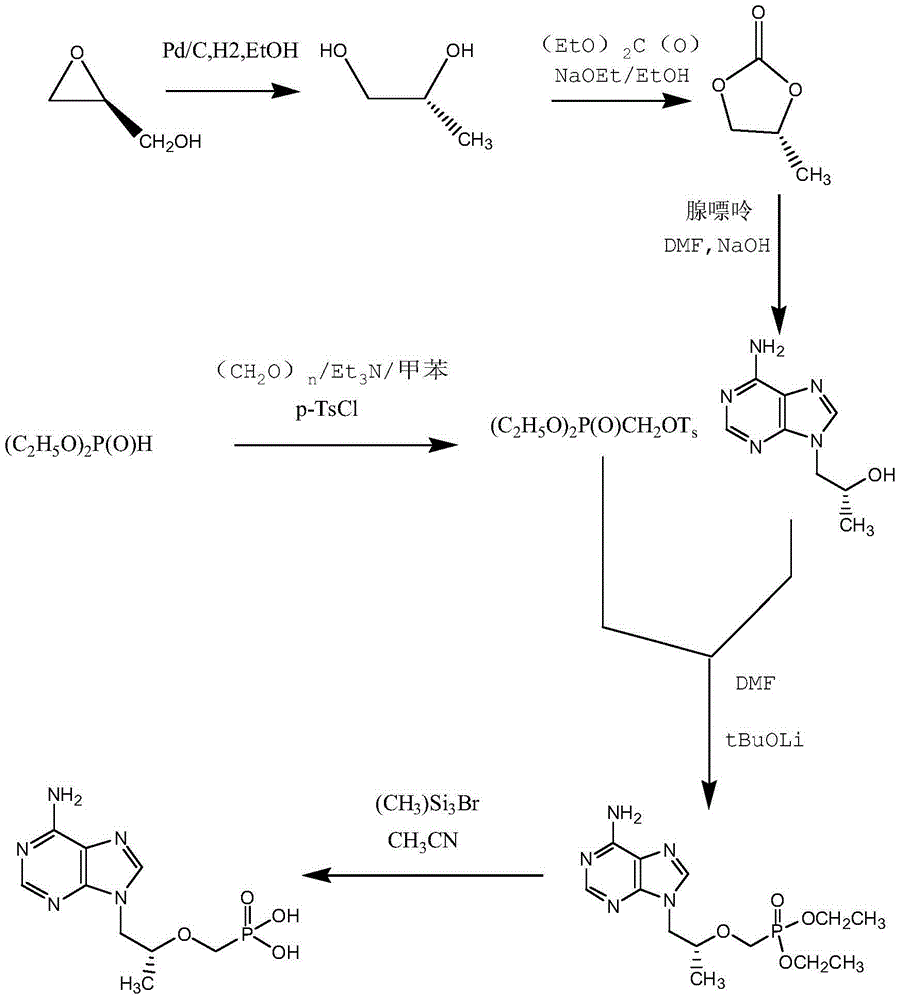
[0033] Preparation of tenofovir
[0034] Reaction formula:
[0035]
Embodiment 1
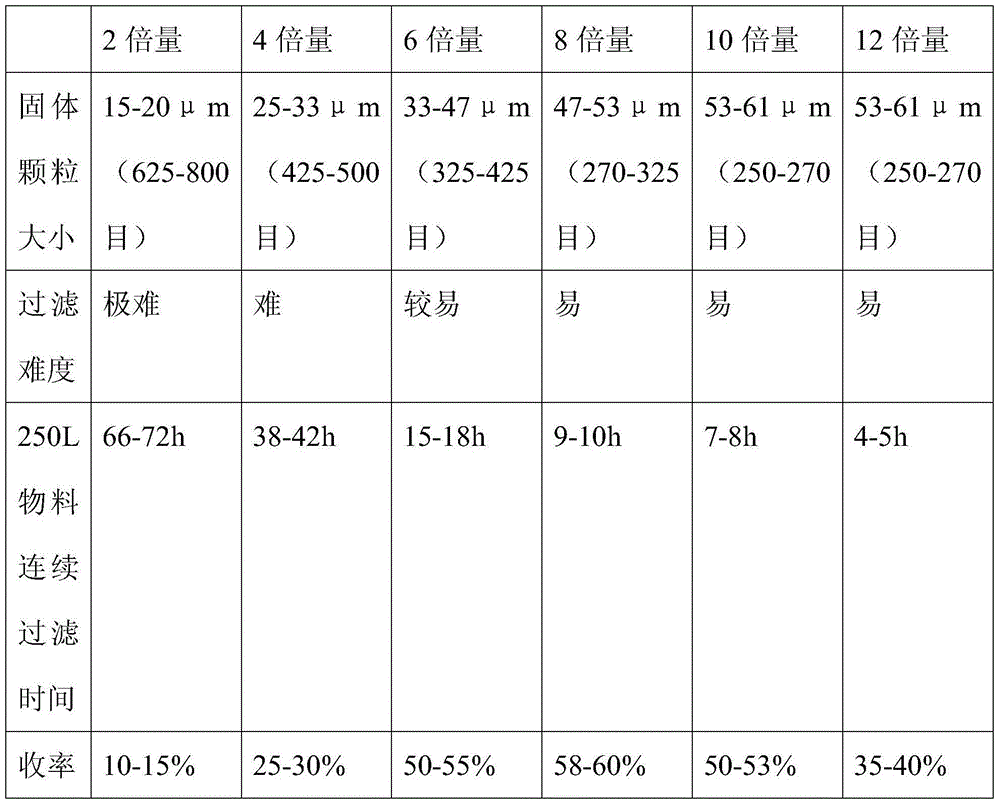
[0037] Feed 20kg of (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine and 160L of acetonitrile. Dissolve at 60°C, add 60kg of trimethylbromosilane dropwise, and react under reflux at 80°C. After the reaction was completed, it was filtered, and the filtrate was concentrated. After the concentration is completed, add 160L of water (the amount of water added: (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine=8:1), let the layers stand, and collect the lower liquid . Add 50% sodium hydroxide to adjust the pH value to ≈3.0, use gradient cooling, first cool down to 20°C for crystallization for 4 hours, then cool down to 10°C for 4 hours, then cool down to 0°C for 8 hours, and stir slowly during the process (stirring speed 100r / min, stirring for 5 minutes every hour). The product was suction filtered, dried, and weighed to obtain tenofovir 10.50kg.
Embodiment 2
[0039]Feed 20kg of (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine and 160L of acetonitrile. Dissolve at 60°C, add 60.00kg of bromotrimethylsilane dropwise, and react under reflux at 80°C. After the reaction was completed, it was filtered, and the filtrate was concentrated. After the concentration is completed, add 120L of water (the amount of water added: (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine=6:1), let the layers stand, and collect the lower liquid . Add 50% sodium hydroxide to adjust the pH value to ≈2.5, use gradient cooling, first cool down to 20°C for crystallization for 4 hours, then cool down to 10°C for 8 hours, then cool down to 0°C for 12 hours, and stir slowly during the process (stirring speed 200r / min, stirring for 5 minutes every hour). Suction filtration, dry, weigh, obtain tenofovir 9.73kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com