Patents
Literature
30 results about "Pralatrexate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
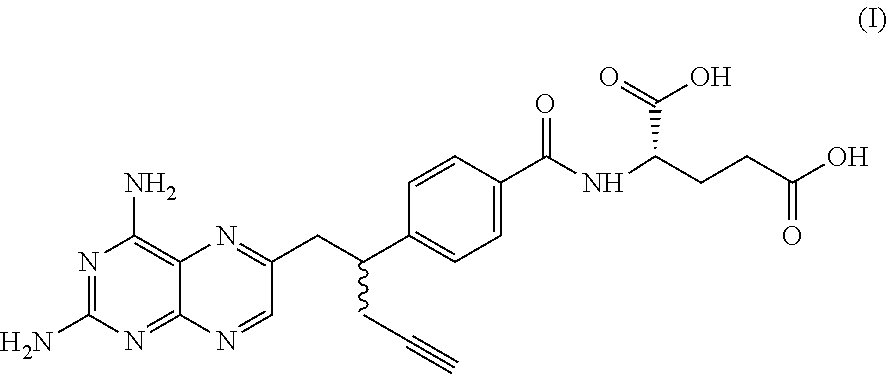
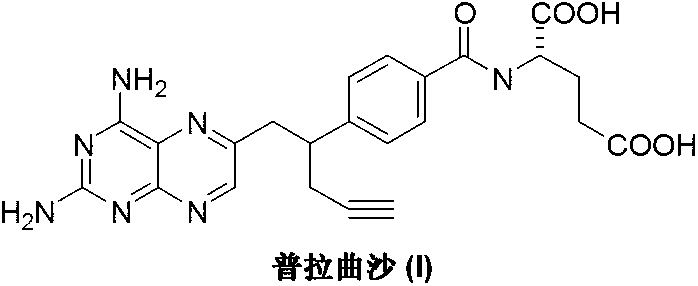
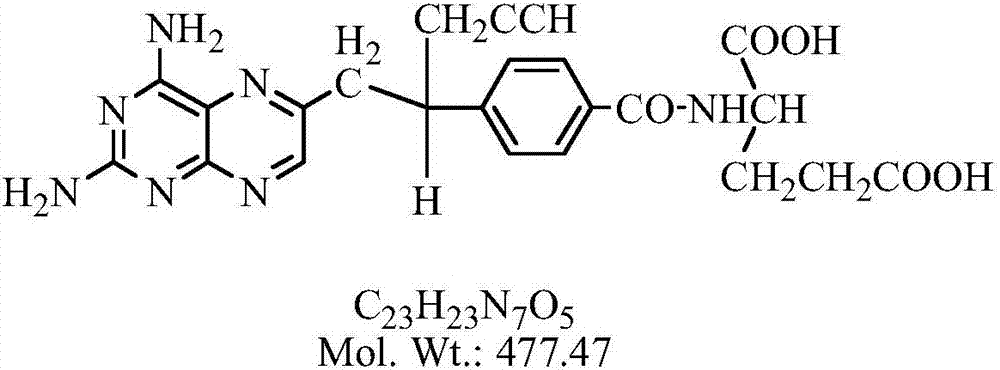
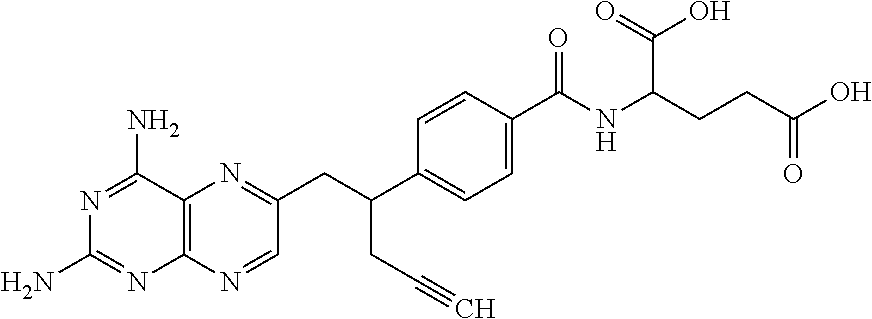
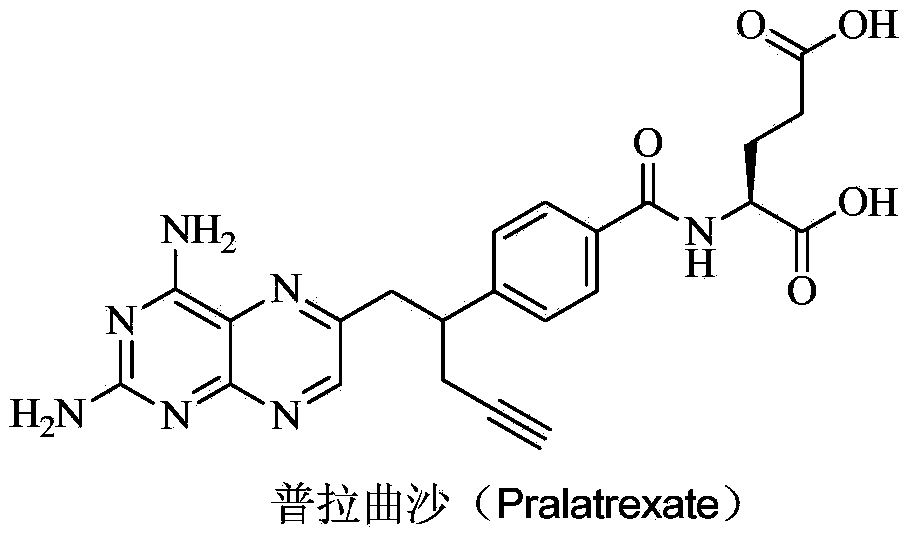
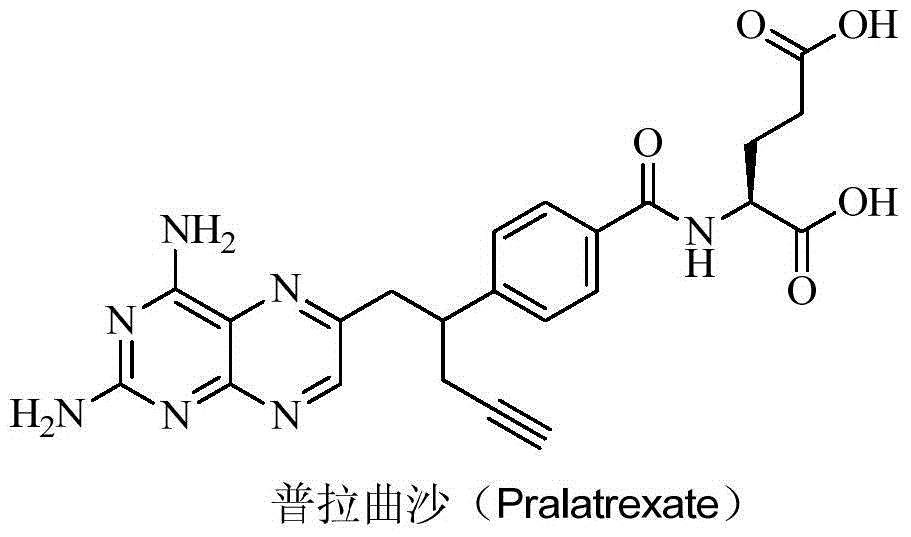
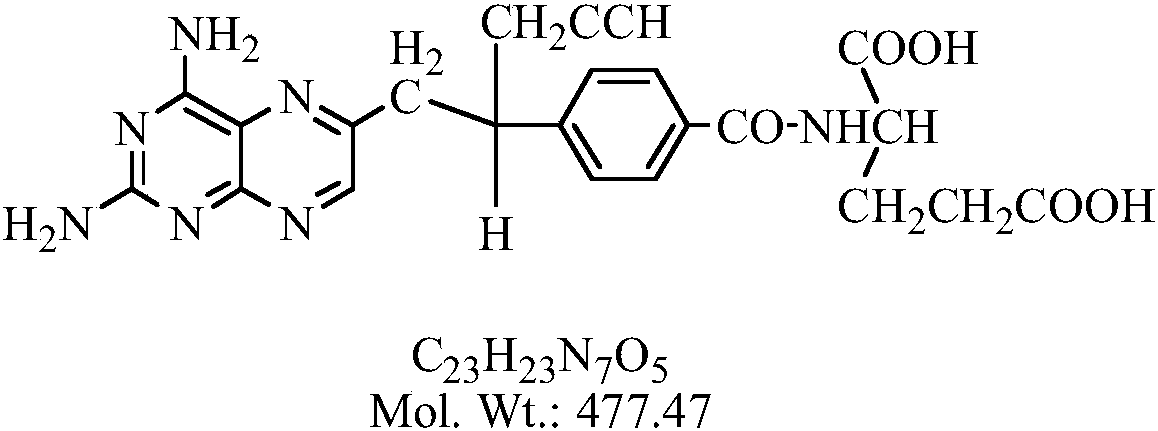
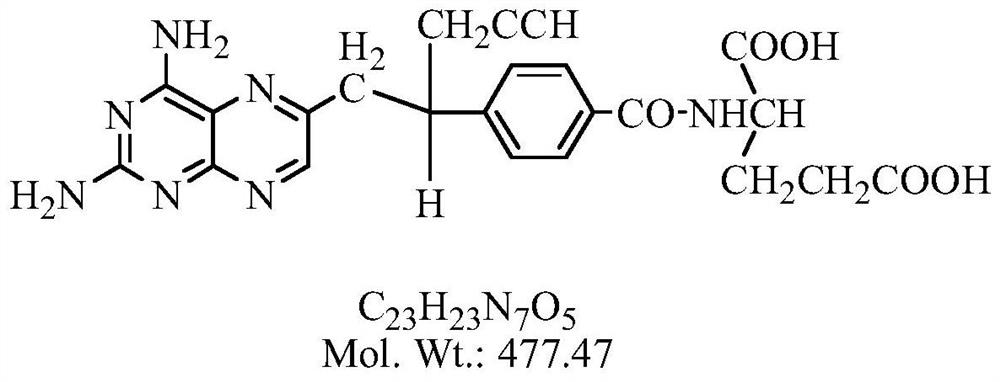
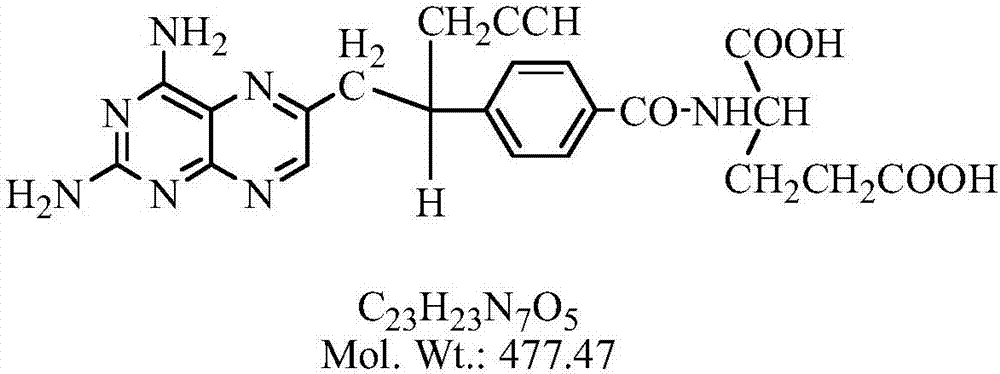
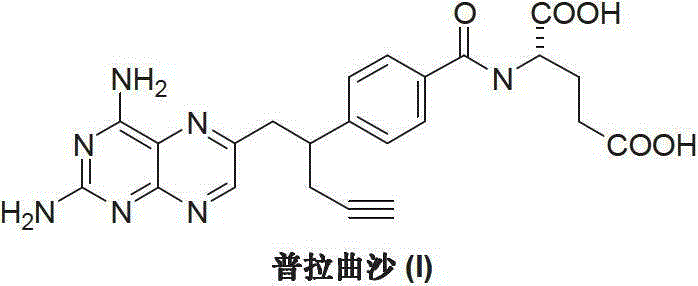
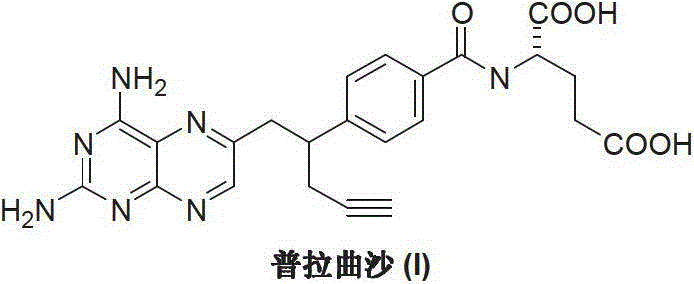
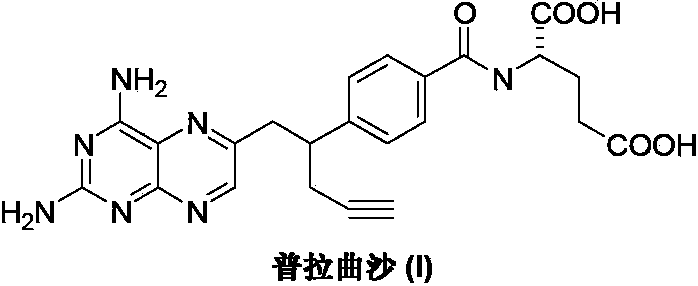
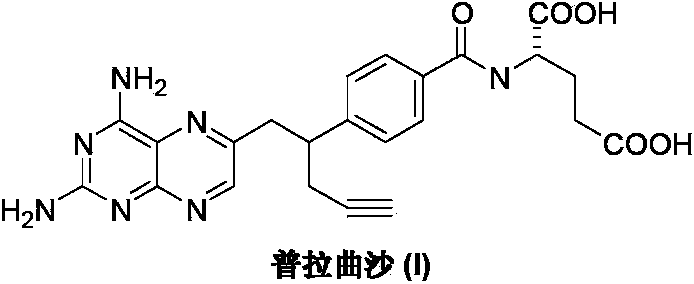
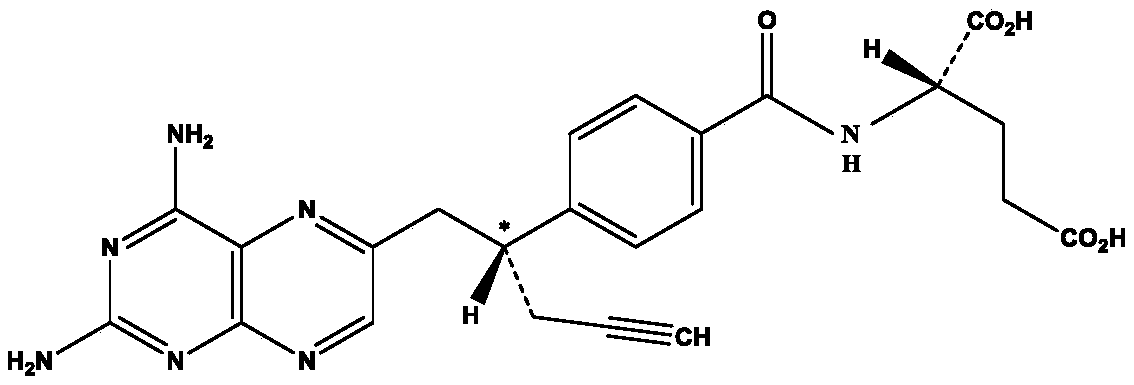
Pralatrexate is used to treat a certain type of cancer known as Peripheral T-Cell Lymphoma (PTCL). It is used when other drugs do not work or the cancer returns after treatment with other drugs.
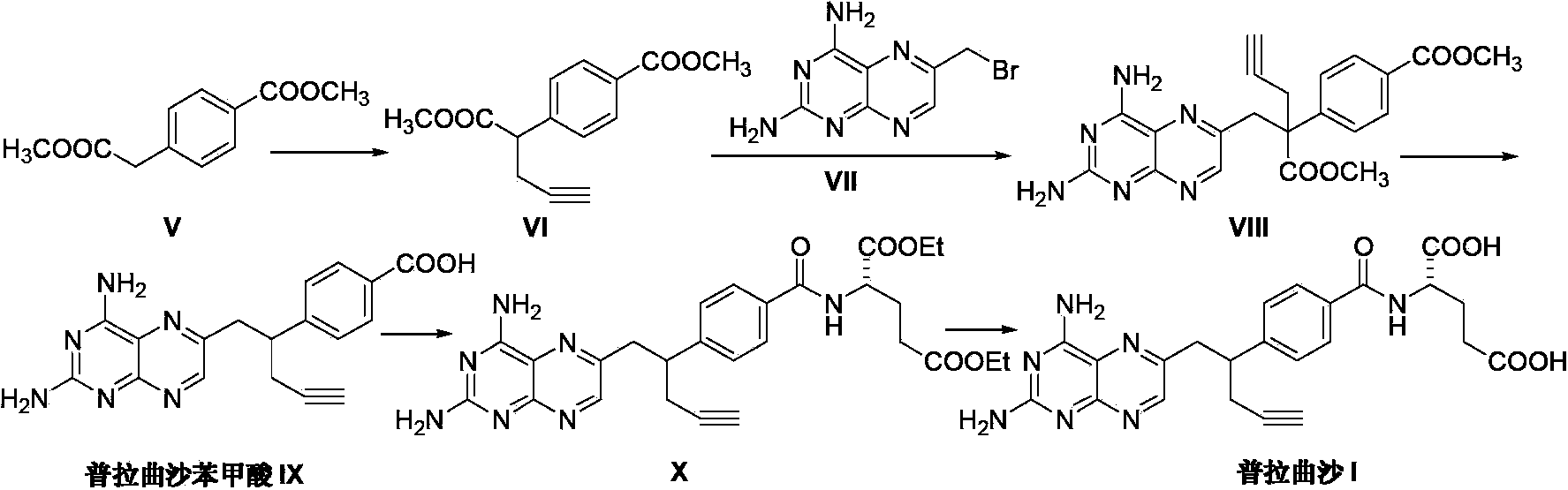
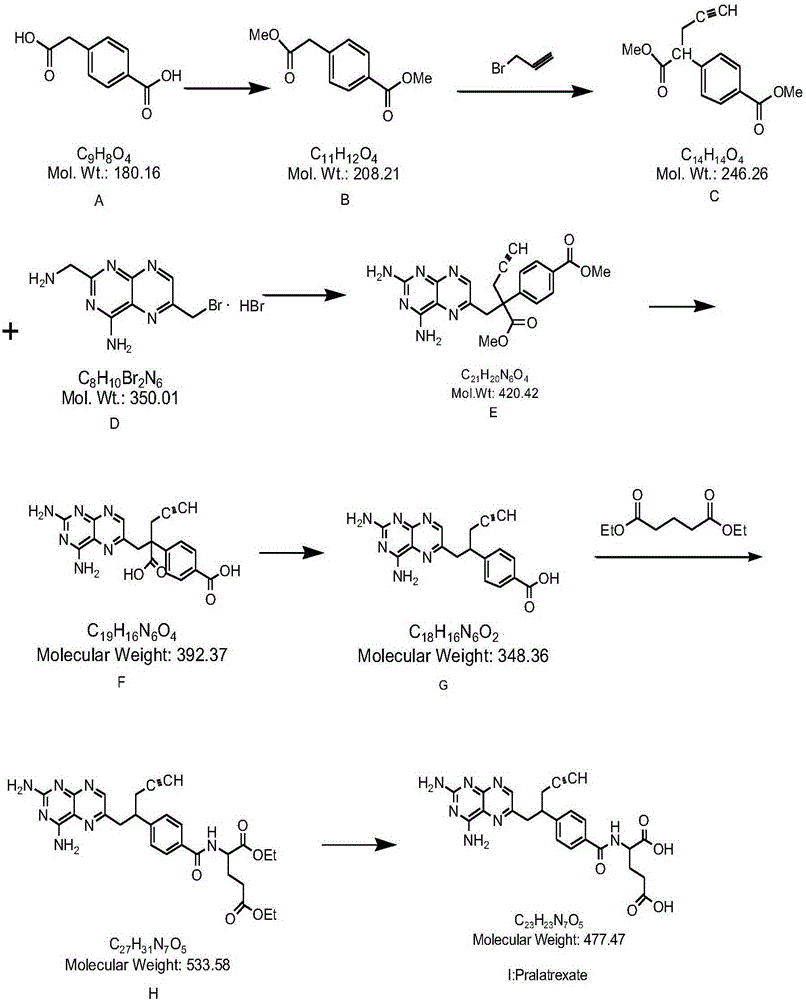
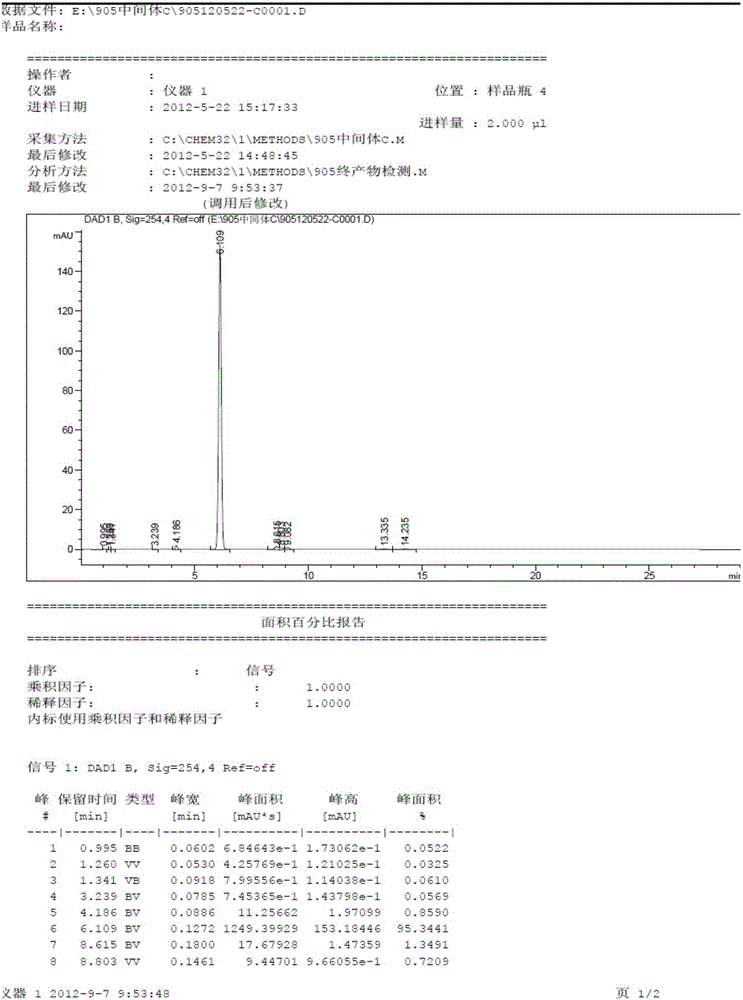
Process for the preparation of pralatrexate
ActiveUS9440979B2Organic compound preparationCarboxylic acid esters preparationPralatrexatePhotochemistry
Owner:FRESENIUS KABI ONCOLOGY LTD
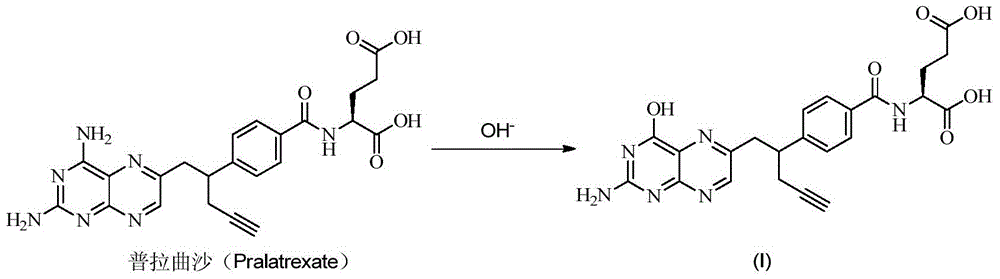
Pralatrexate preparation method
InactiveCN103275080AEase of industrial productionRaw materials are easy to getOrganic chemistryPteridine synthesisPralatrexate
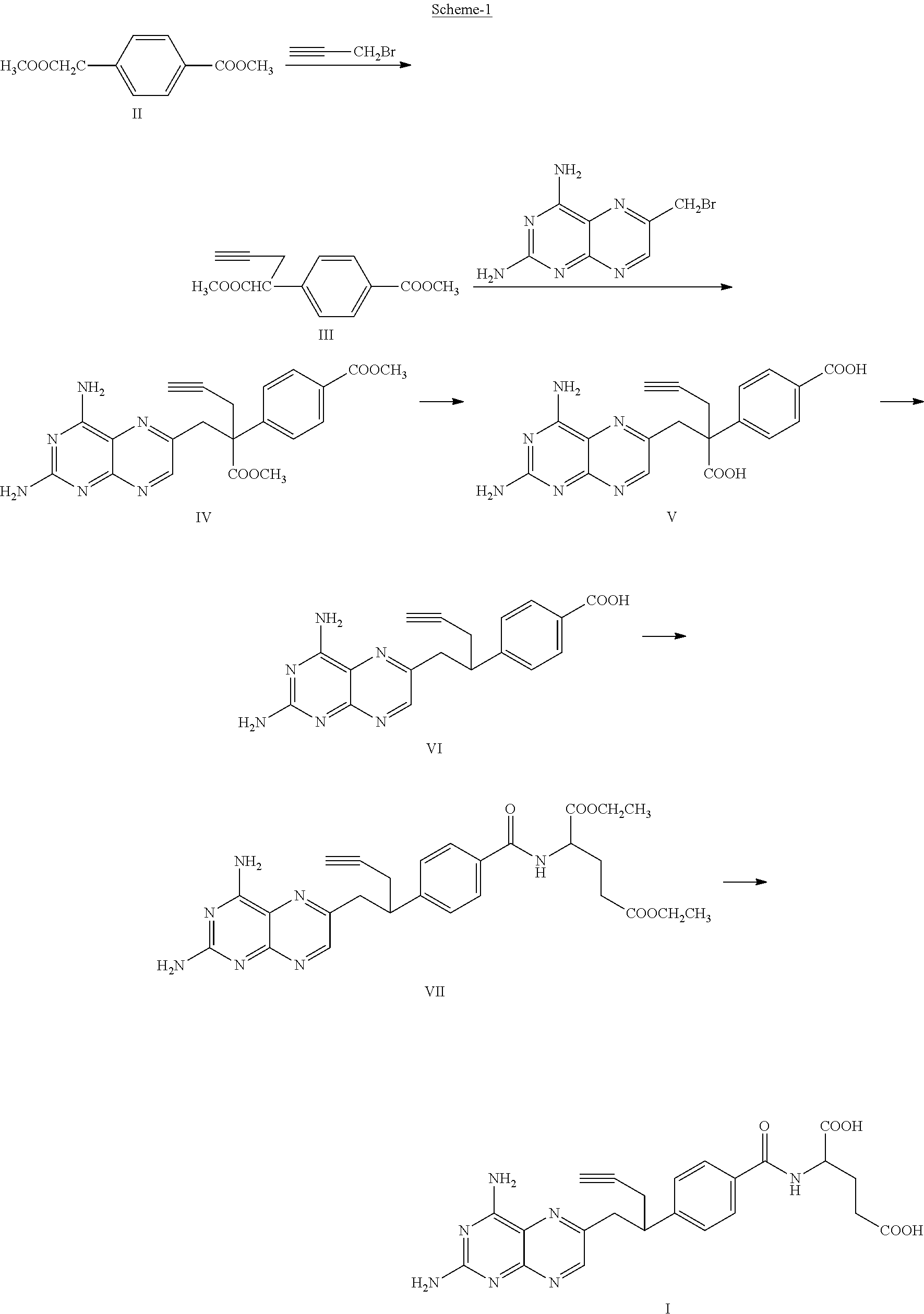
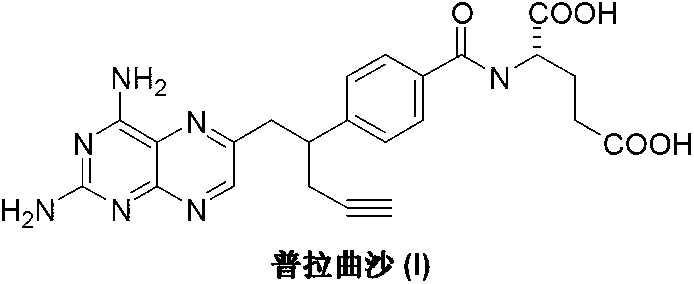
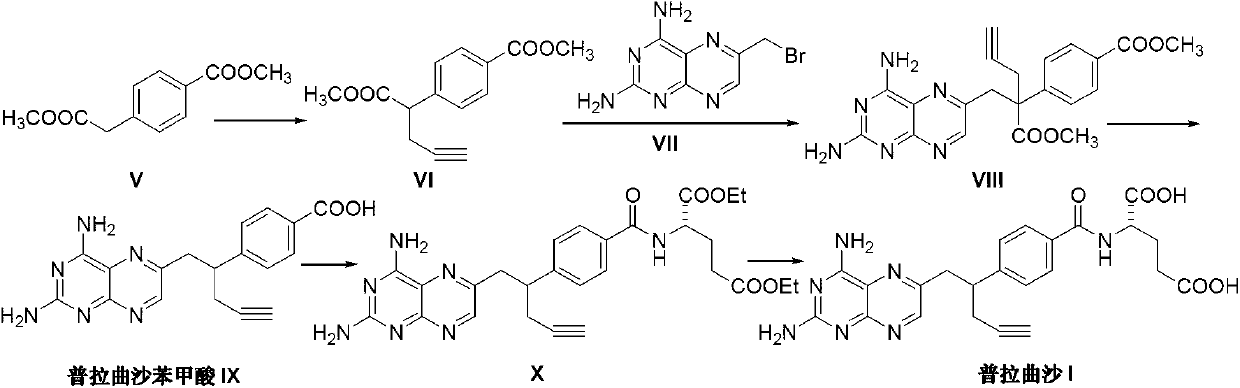
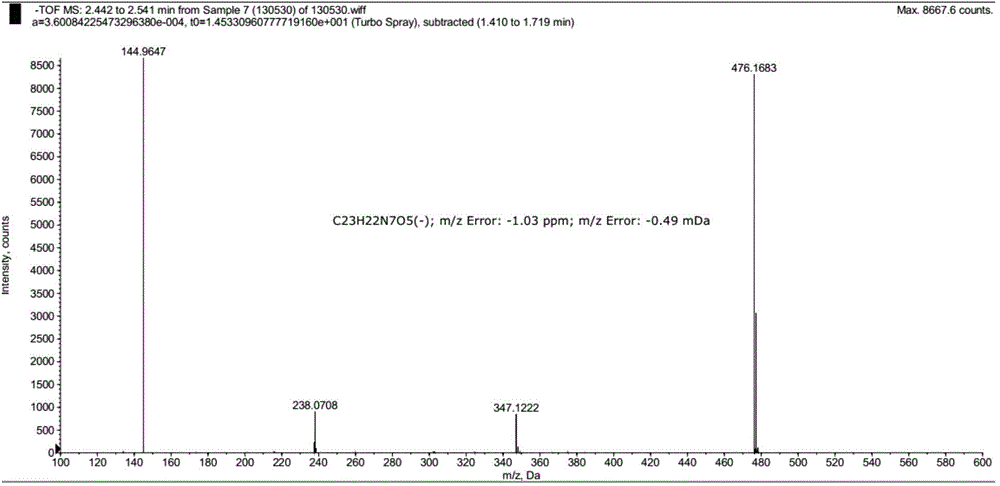
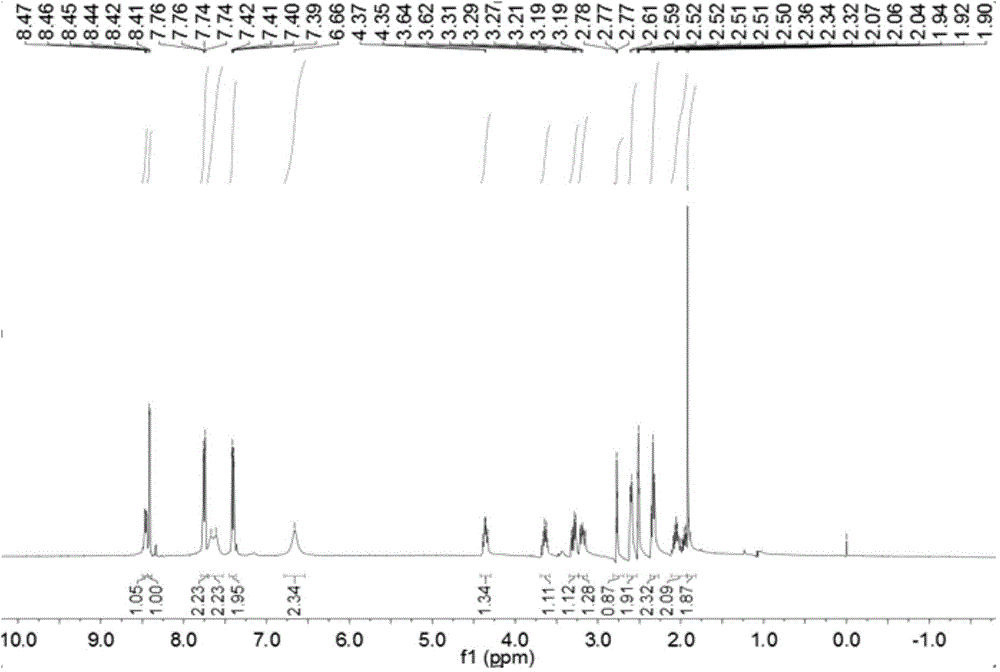
The invention discloses a pralatrexate (I) preparation method, which comprises the following preparation steps: performing cyclization reaction on dialkyl N-[4-(1-(2-propinyl)-3,4-dioxo-n-butyl)benzoyl]-L-glutamate (II) and 2,4,5,6-tetraminopyrimidine (III) under oximation conditions to generate an intermediate compound alkyl N-[4-[1-(2,4-diamino-6-pteridine)methyl-3-butynyl]benzoyl]-L-glutamate (IV); and performing hydrolysis reaction on the intermediate compound (IV) to prepare pralatrexate (I). The preparation method is accessible in raw materials, simple in process, economic, environment-friendly and suitable for industrial production.
Owner:安徽金太阳生化药业有限公司
Pralatrexate preparation method
InactiveCN104628728AAvoid high energy consumptionAvoid separationOrganic chemistryBenzoic acidPteroic acid
The present invention discloses a practical synthesis process of a new anticancer drug pralatrexate. According to the present invention, 10-propargyl-10-methoxycarbonyl-4-deoxy-4-amino-10-deaza pteroic acid methyl ester is adopted as a starting raw material, a saponification reaction is performed to obtain 4-(2-carboxy-1-(2,4-diaminopteridine-6-yl)pent-4-yn-2-yl)benzoic acid, the 4-(2-carboxy-1-(2,4-diaminopteridine-6-yl)pent-4-yn-2-yl)benzoic acid is subjected to decarboxylation to obtain 4-(1-(2,4-diaminopteridine-6-yl)pent-4-yn-2-yl)benzoic acid, the 4-(1-(2,4-diaminopteridine-6-yl)pent-4-yn-2-yl)benzoic acid reacts with L-diethyl glutamate to obtain 10-propargyl-10-deaza aminopterin diethyl ester, and finally a saponification reaction is performed to obtain the target product pralatrexate, wherein the green, high yield and convenient synthesis of the pralatrexate is completed, the final product can achieve the level from several grams to tens of grams, the purity is more than or equal to 90%, and the good industrial application prospects are provided.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
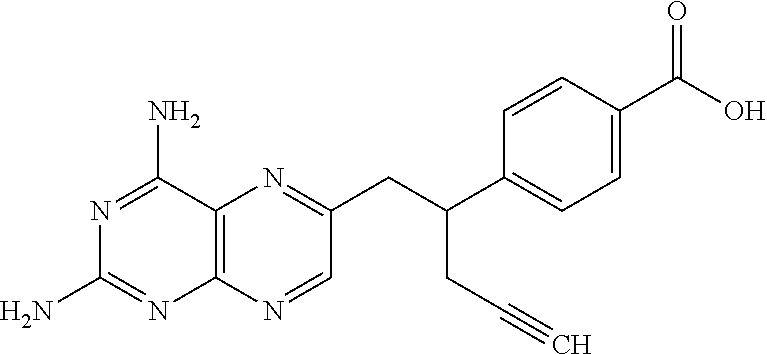
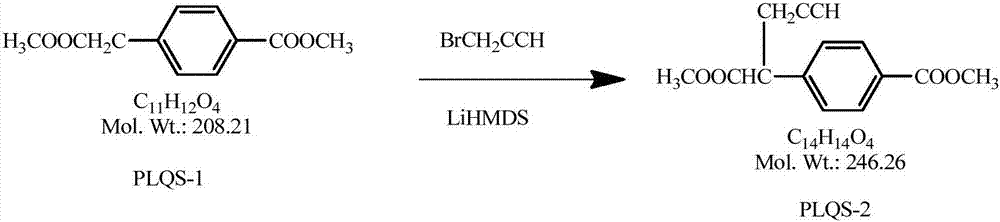
4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof
ActiveCN103274943AEasy to manufactureImprove economyOrganic compound preparationCarboxylic acid esters preparationBenzaldehydeMetal catalyst
The invention discloses a novel medical compound 4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate (I) and a preparation method thereof. The preparation method comprises the following steps of carrying out an addition reaction on 4-carbalkoxy benzaldehyde (II) and 3-propargyl bromide (III) to generate 4-(1-hydroxyl-3-butyne) benzoate (IV); and carrying out a coupled reaction on an intermediate (IV) and pyruvaldehyde dialkyl acetal (V) after being subjected to enolization under the action of a metal catalyst to form the 4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate (I). According to the intermediate (I) and the preparation method thereof, a novel preparation way for the antineoplastic pralatrexate is provided and the economic and technological development of the pralatrexate is promoted.
Owner:临沂经开财金投资发展有限公司
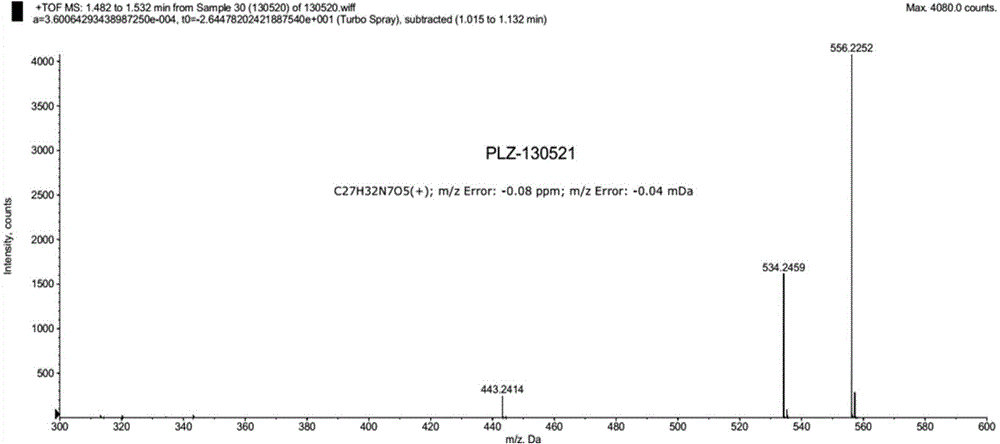
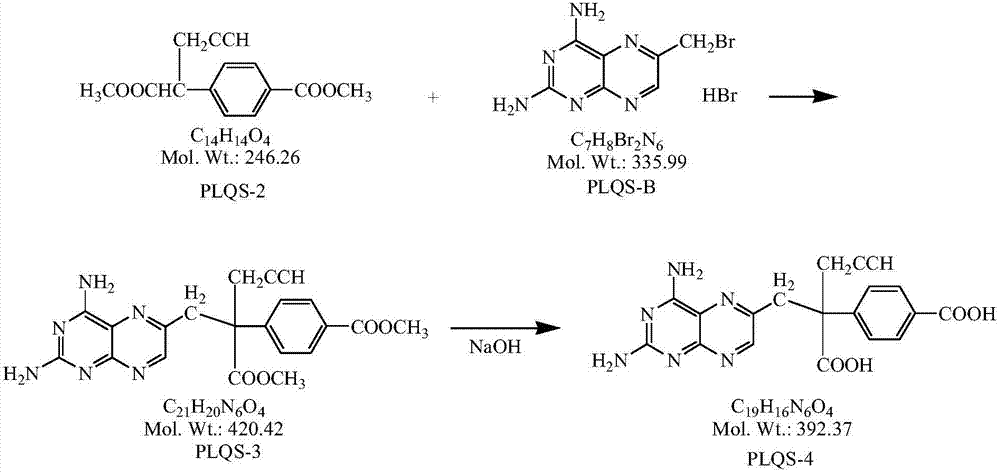
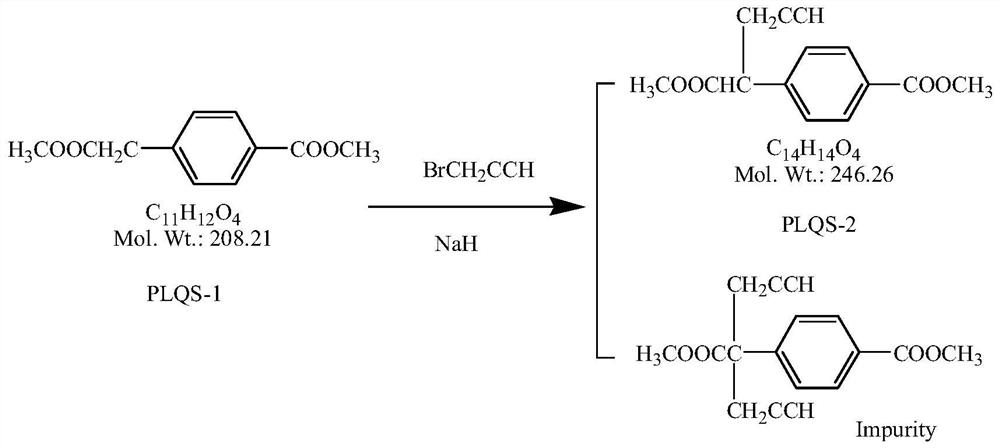
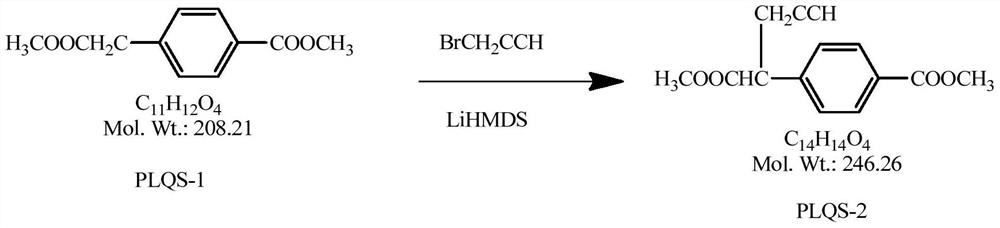
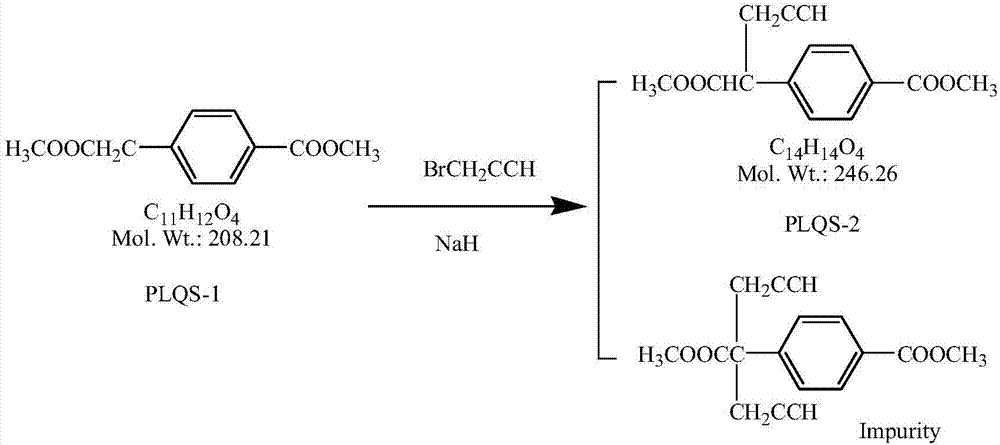
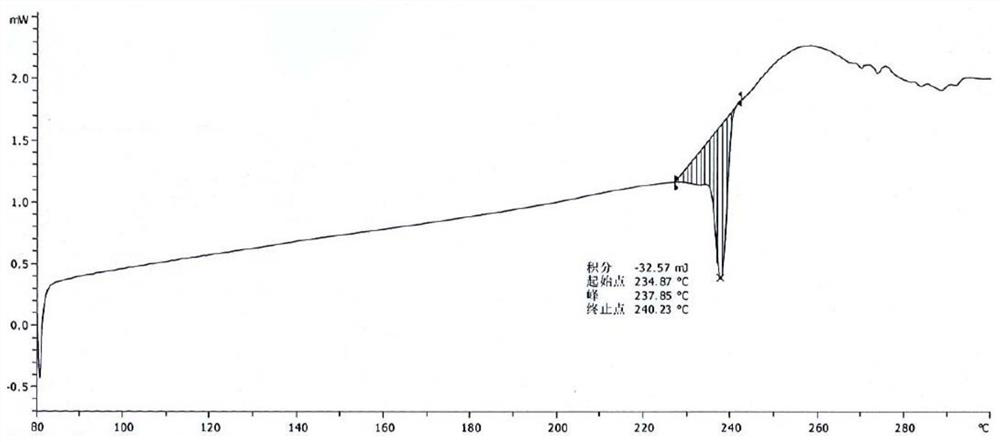
Preparation method for high-purity pralatrexate intermediate
The invention specifically relates to a preparation method for high-purity pralatrexate intermediate, belonging to the technical field of medicine. The preparation method is based on preparation of PLQS-3 and comprises the following steps: preparing crude PLQS-3 by using a crystallization solvent; then heating and washing the crude PLQS-3 with a single solvent, and carrying out filtering while the crude PLQS-3 is still hot so as to obtain high-purity PLQS-3; subjecting the PLQS-3 to hydrolysis with an appropriate amount of inorganic alkali liquor at a certain temperature; adding excess hydrochloric acid into a hydrolysis solution to obtain a hydrochloride; and refining the hydrochloride with a solvent such as anhydrous ethanol to obtain high-purity PLQS-4 hydrochloride. According to the invention, the purity of PLQS-4 is improved through refining of the crude PLQS-3; and the preparation method can be well used in the production of pralatrexate.
Owner:SHANDONG NEWTIME PHARMA
Process for pralatrexate
ActiveUS9783542B2Organic compound preparationCarboxylic acid esters preparationDimethyl terephthalatePralatrexate
Owner:HETERO RES FOUND
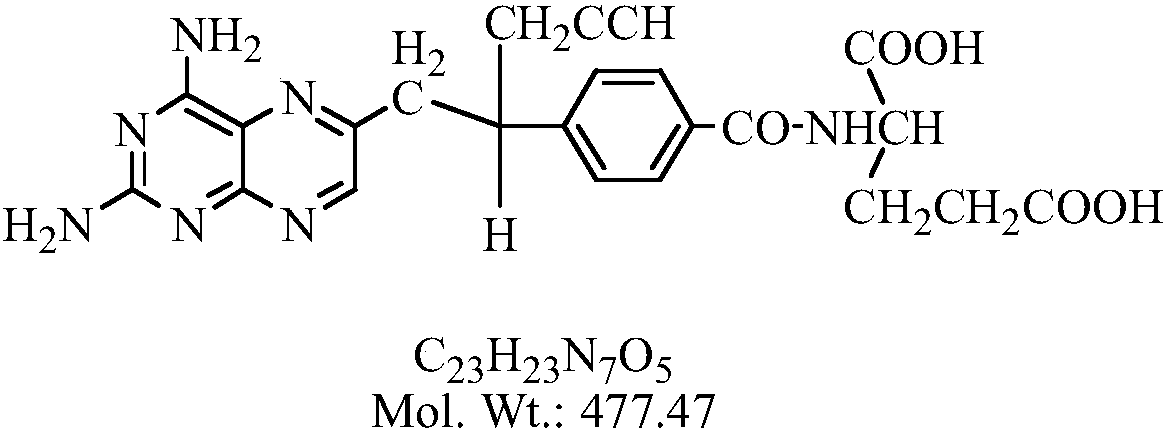
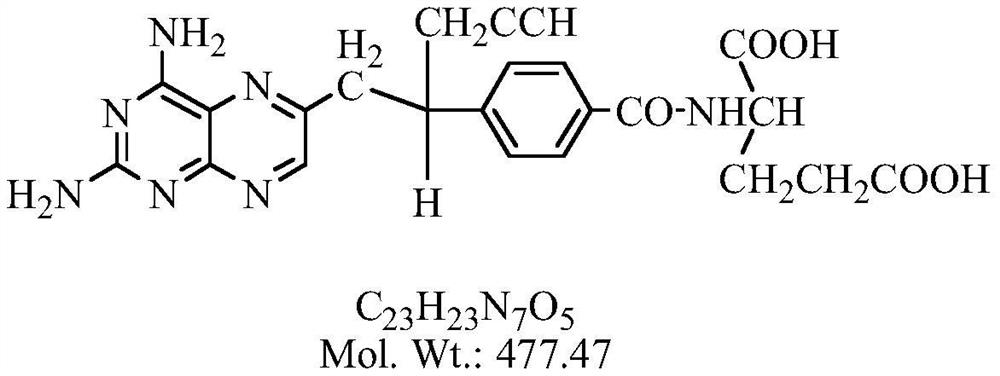
Pralatrexate degradation impurity and preparation method thereof
ActiveCN103588775AEfficient productionPremium quality gets producedOrganic chemistryPteridine synthesisBenzoic acid
The invention relates to a pralatrexate degradation impurity and a preparation method thereof. Specifically, the formula (I) of the pralatrexate degradation impurity is N-[4-1- [(2-amino-4-hydrox-6-pteridine) methyl]-3-butine-1-group] benzoyl]-L-glutamate. An N-[4-1- [(2-amino-4-diamido-6-pteridine) methyl]-3-butine-1-group] benzoic acid compound is subjected to substitution, condensation and hydrolysis, and a target compound is obtained, so that the pralatrexate degradation impurity is synthesized. According to the method, the compound of the formula (I) is chemically synthesized for the first time, and the obtained target compound can be separated efficiently and rapidly.
Owner:连云港恒运药业有限公司
Small-molecule inhibitor of influenza virus
ActiveCN113679724ALow toxicityOrganic active ingredientsDigestive systemPralatrexatePharmaceutical Substances
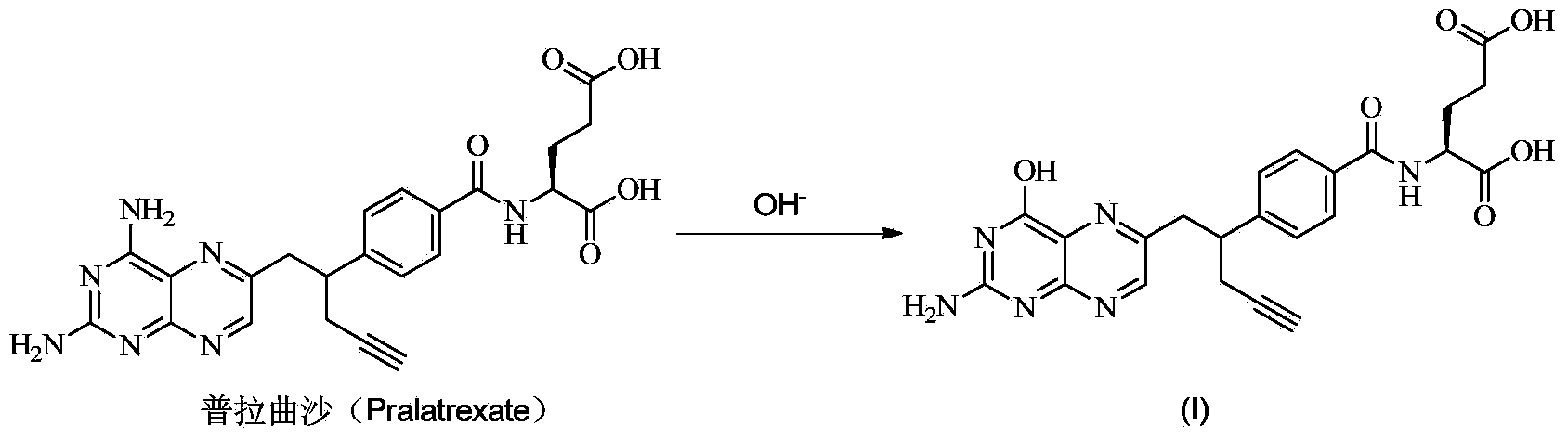
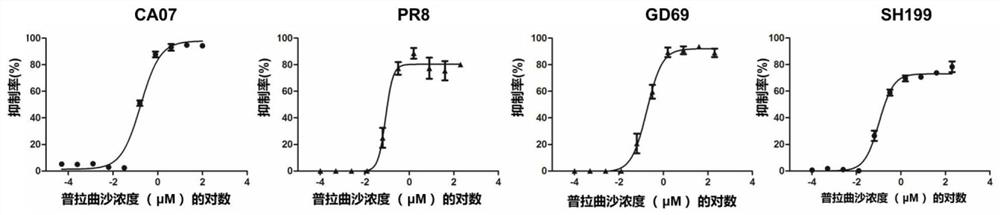
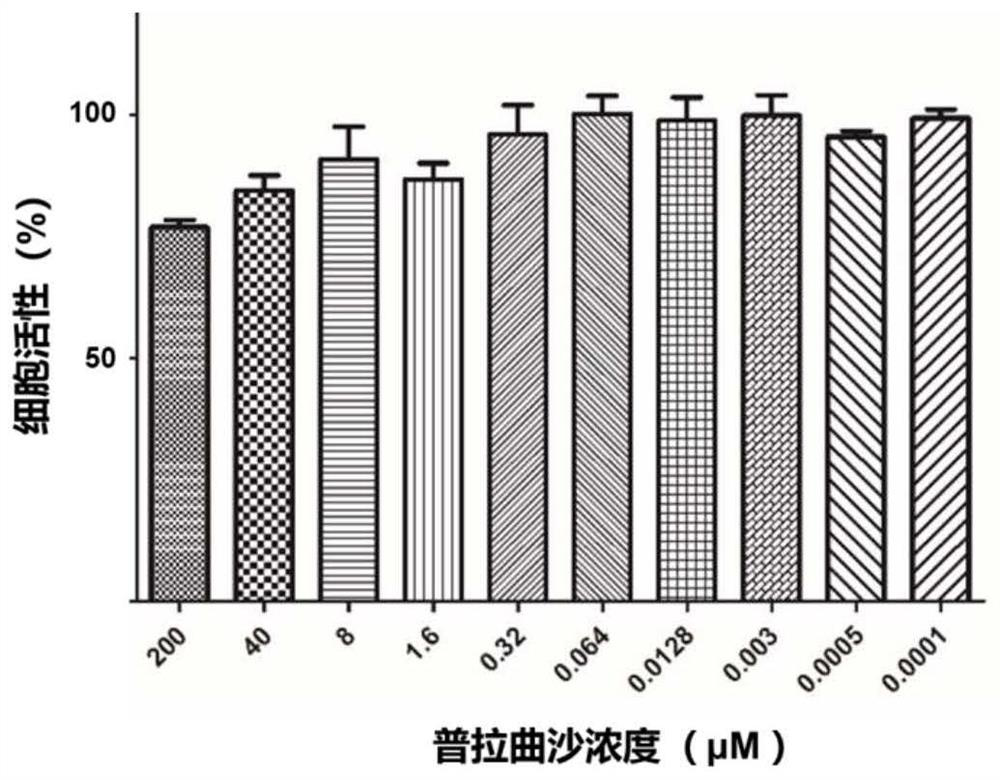
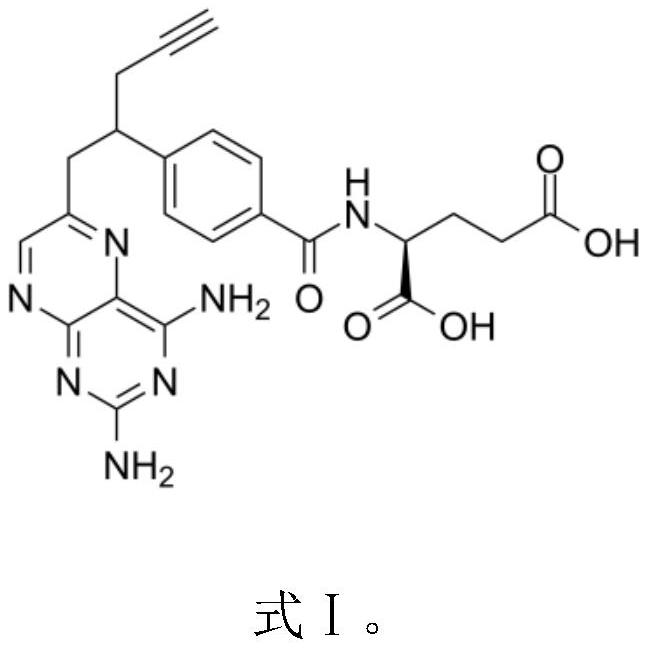
The invention discloses a small-molecule inhibitor of influenza virus. The small-molecule inhibitor is pralatrexate, the structural formula of the pralatrexate is shown as a formula I, and the small-molecule inhibitor can inhibit influenza viruses, specifically influenza A viruses such as H1N1, H5N1 or H5N6 subtype influenza viruses. The research finds that the small-molecule inhibitor has a remarkable inhibition effect on H1N1, H5N6 and H5N1 subtype influenza viruses, and the compound shown in the formula I has broad-spectrum anti-influenza A virus potential preliminarily; meanwhile, the toxicity of the small-molecule inhibitor to cells is small, the CC50 of the small-molecule inhibitor to MDCK cells is larger than 200 mu M, the CC50 / EC50 ratio (namely the selectivity index SI) is larger than 200, and the small-molecule inhibitor can be used as a safe and effective anti-influenza virus medicine.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Preparation process for high-purity pralatrexate intermediate
InactiveCN110227281AImprove cooling effectPromote precipitationSolution crystallizationHeat exchange cooling cystallizationPralatrexateDissolution
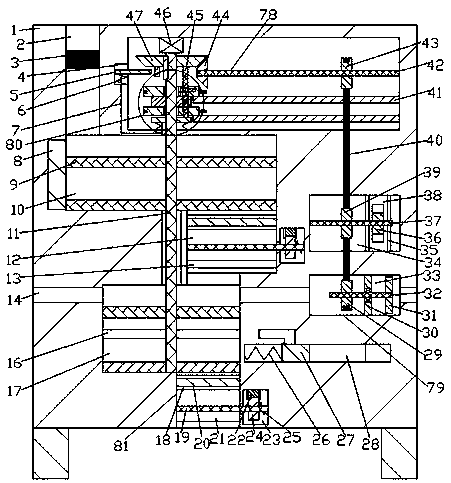
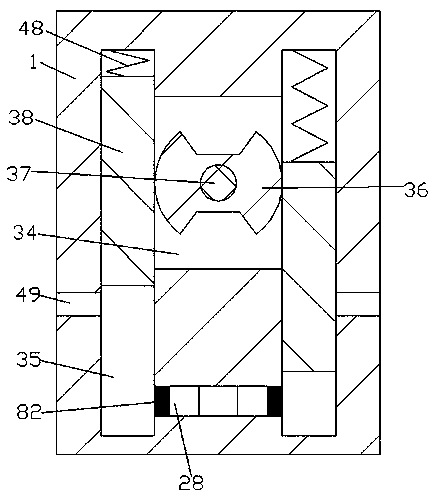
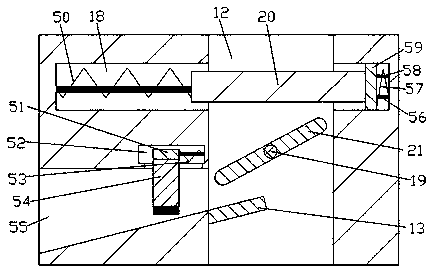
The invention discloses a preparation process for a high-purity pralatrexate intermediate. A purification tank is used in the process; a driving chamber is arranged in the purification tank, and the purification tank is internally provided with a driving mechanism for powering the operation of a device used in the process; a dissolution chamber and a crystallization chamber are also arranged in the purification tank; the lower end walls of the dissolution chamber and the crystallization chamber are both internally provided with filtering mechanisms; an air inlet mechanism is arranged in the right end wall of the crystallization chamber; the driving chamber is internally provided with a control mechanism for controlling the operation of the filtering mechanism and the air inlet mechanism. When the device is used for purification of a pralatrexate intermediate, the cooling of a dissolved solution is accelerated in the manners of rotary stirring, bubble stirring and acceleration of gas flow in the crystallization chamber, so the precipitation of crystals in the dissolved solution is accelerated, which thus accelerates purification efficiency; the device carries out both dissolving andcrystallization, so the degree of automation is increased; and dissolving is accelerated by mechanical transmission.
Owner:嘉兴冷暮熙贸易有限公司
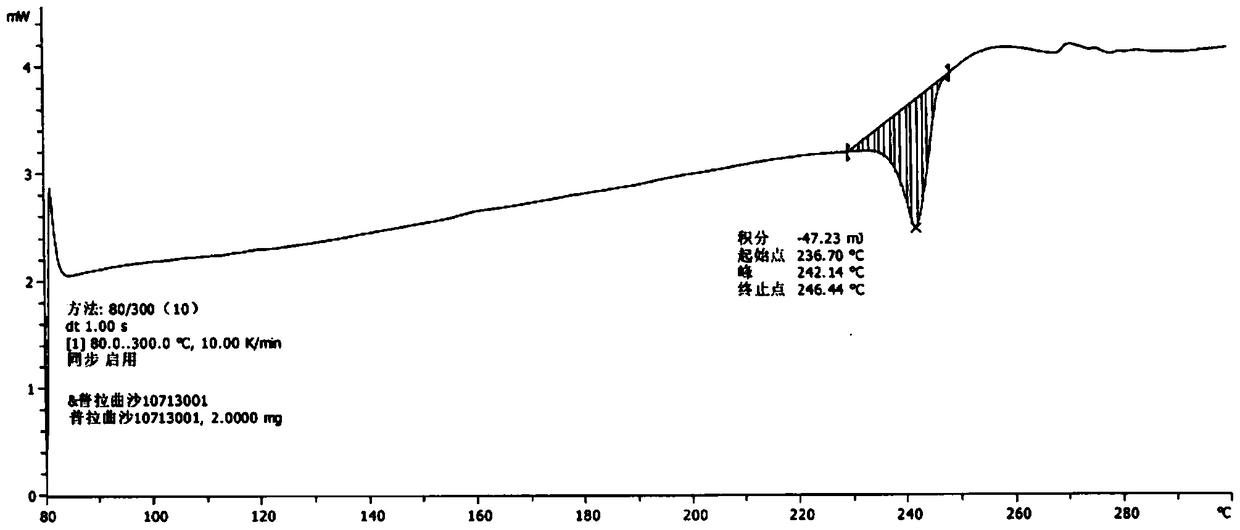
A kind of crystal form of pralatrexate and preparation method thereof
ActiveCN104628727BHigh purityHigh crystallinityOrganic active ingredientsOrganic chemistryPralatrexateSolvent
The invention provides a pralatrexate crystal form and a preparation method thereof, and concretely relates to a pralatrexate E crystal form, a preparation method thereof, a crystallized composition containing the pralatrexate E crystal form, and a medicinal composition containing the pralatrexate E crystal form. The pralatrexate E crystal form prepared in the invention has the advantages of high purity, high degree of crystallization, and good stability; and the preparation method of the pralatrexate E crystal form has the advantages of simplicity, cheap and easily available solvent, mild crystallization conditions, and suitableness for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Pralatrexate degraded impurities and preparation method thereof
ActiveCN103588775BEfficient productionPremium quality gets producedOrganic chemistryChemical synthesisBenzoic acid
The invention relates to a pralatrexate degradation impurity and a preparation method thereof. Specifically, the formula (I) of the pralatrexate degradation impurity is N-[4-1- [(2-amino-4-hydrox-6-pteridine) methyl]-3-butine-1-group] benzoyl]-L-glutamate. An N-[4-1- [(2-amino-4-diamido-6-pteridine) methyl]-3-butine-1-group] benzoic acid compound is subjected to substitution, condensation and hydrolysis, and a target compound is obtained, so that the pralatrexate degradation impurity is synthesized. According to the method, the compound of the formula (I) is chemically synthesized for the first time, and the obtained target compound can be separated efficiently and rapidly.
Owner:连云港恒运药业有限公司
Refining method of pralatrexate intermediate
ActiveCN108069971AHigh purityEasy to recycleOrganic chemistryChromatographic separationActivated carbon
The invention belongs to the technical field of medicine and particularly relates to a refining method of a pralatrexate intermediate. The method comprises the following steps: adding a crude productof an intermediate 10-propargyl-10-deazaaminopterin dimethyl ester (PLQS-6) to a refining solvent to be heated and dissolved; performing activated carbon decoloration and hot filtration; cooling, stirring and crystallizing the filtrate; and washing the filter cake with the refining solvent so as to obtain a high-purity pralatrexate intermediate PLQS-6. The used refining solvent is a low-boiling-point solvent, so that the problem of excessive solvent residues such as DMF (dimethyl formamide) and DMSO (dimethyl sulfoxide) is avoided, the refining solvent can also be recycled, and the cost is saved. The method provided by the invention has the advantages that application of column chromatography separation is avoided, the yield is improved, the whole technological operation is simplified, theoperation method is simple, the yield and the purity of the obtained pralatrexate intermediate are higher, and the method is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
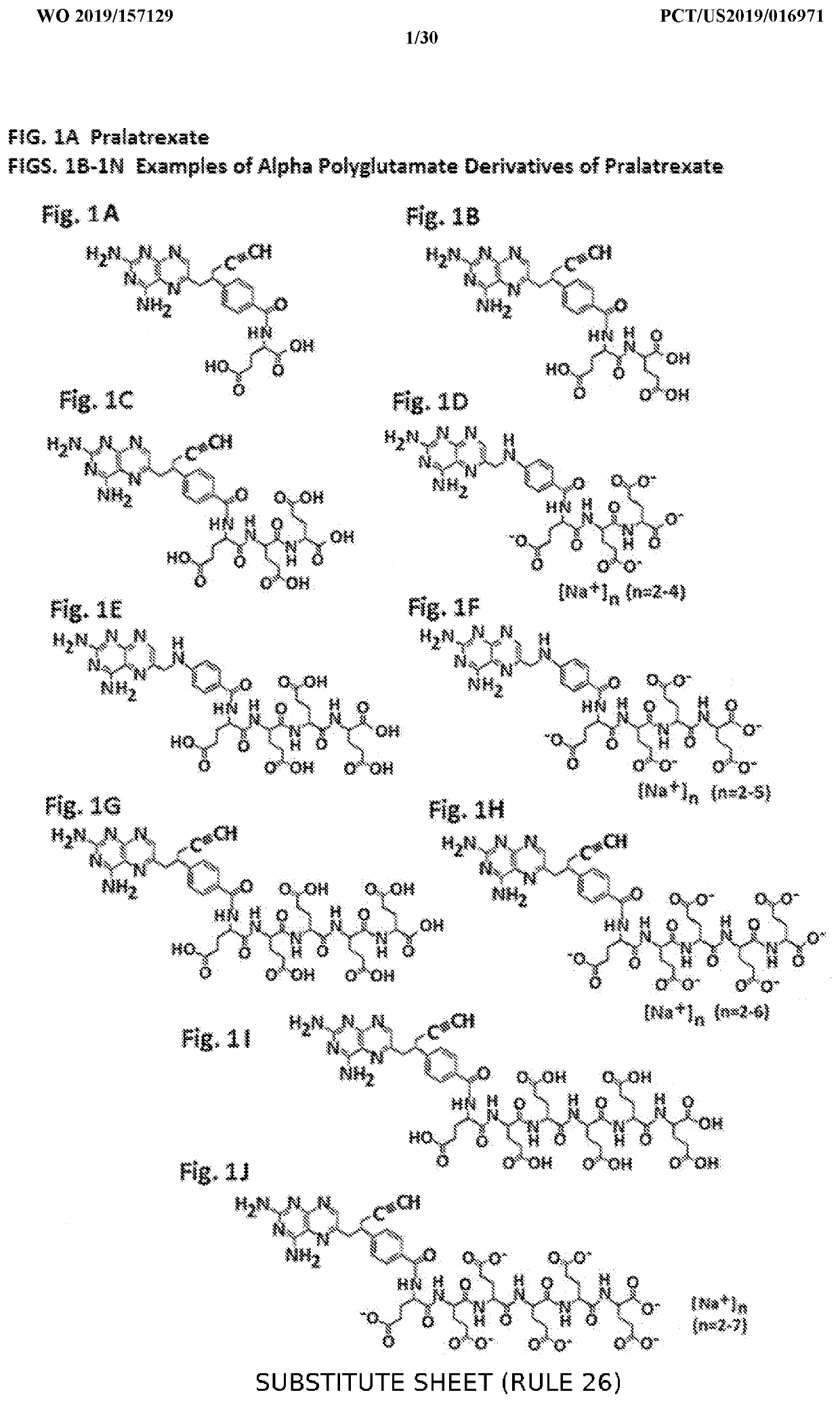
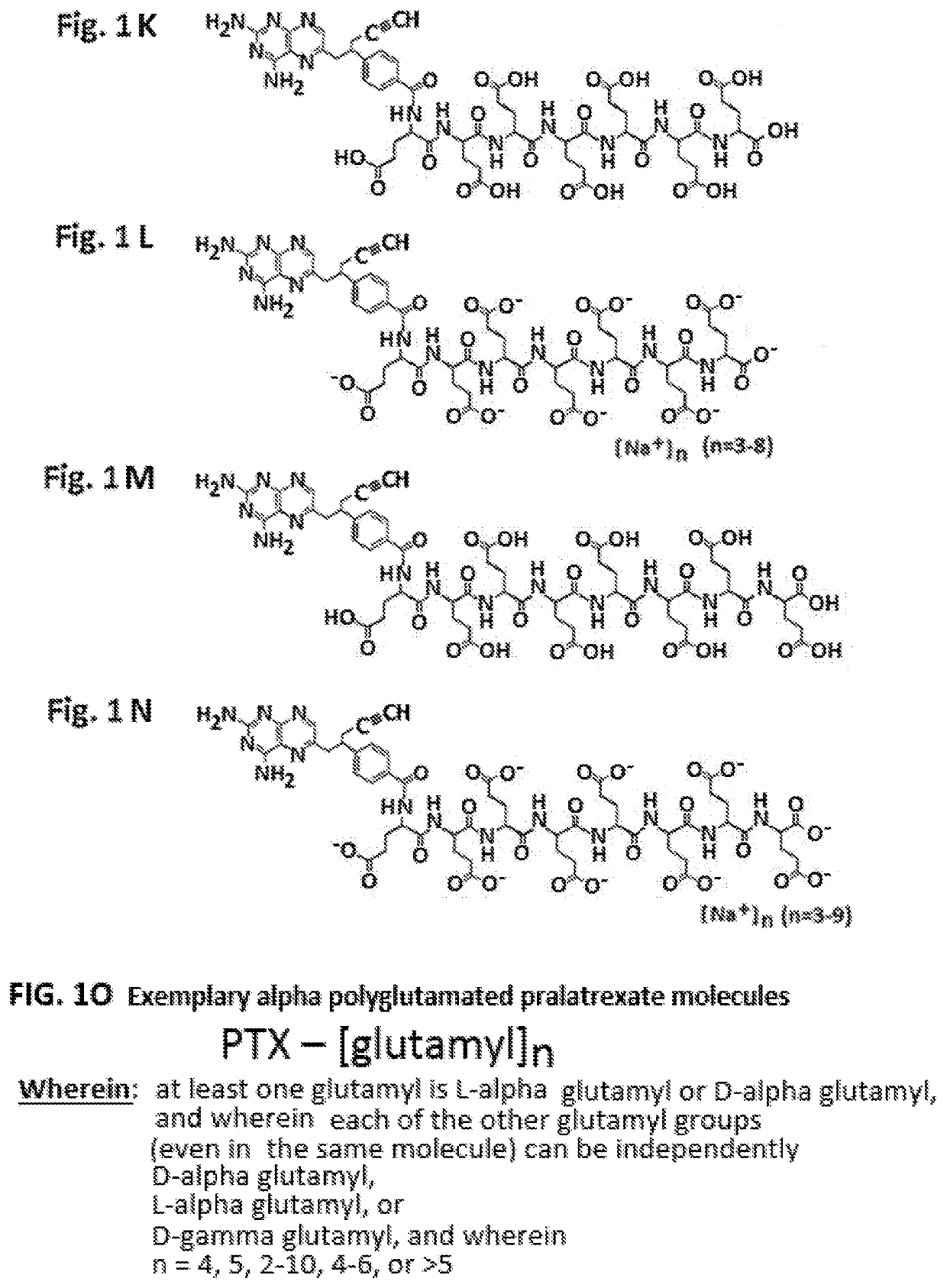
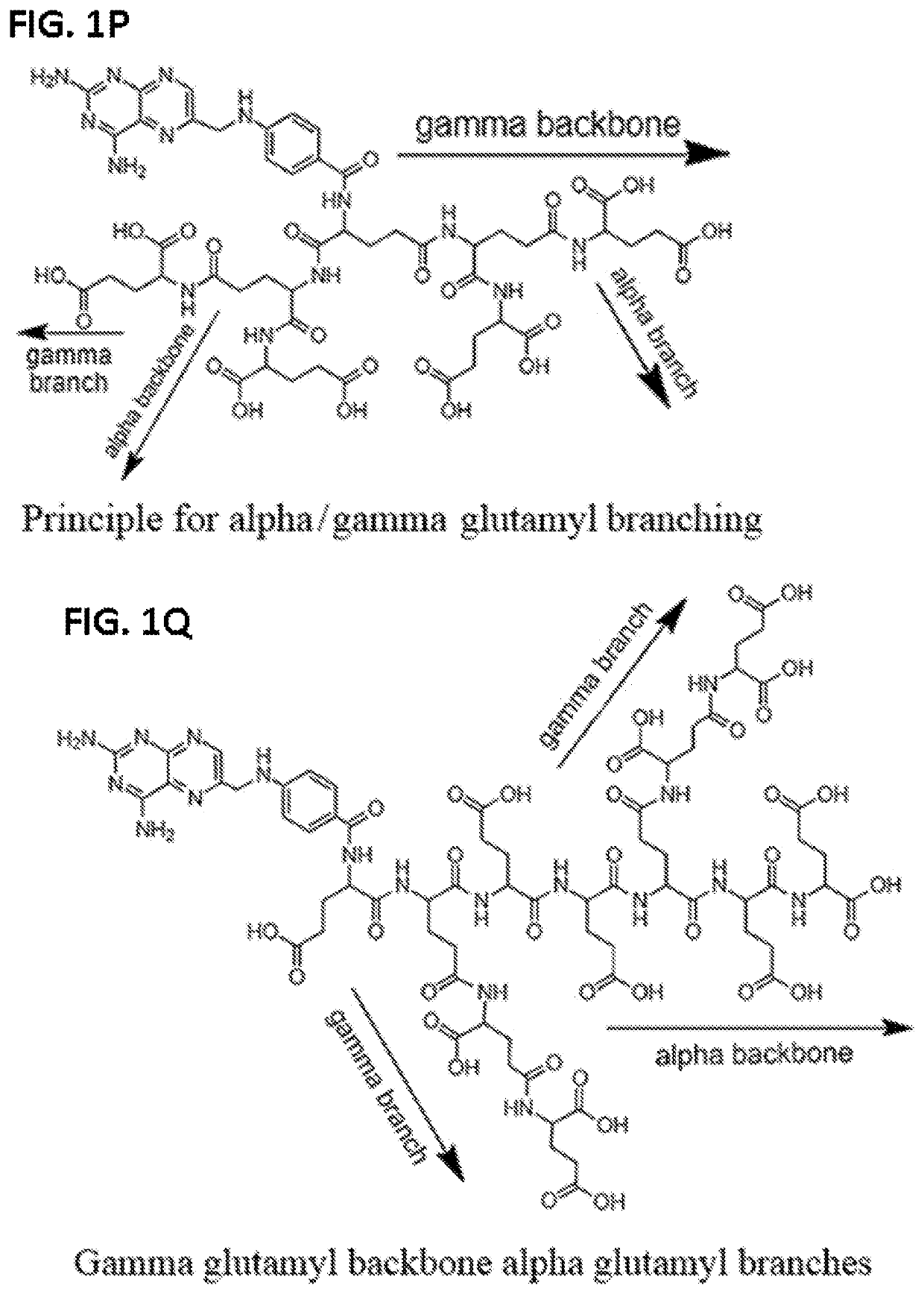
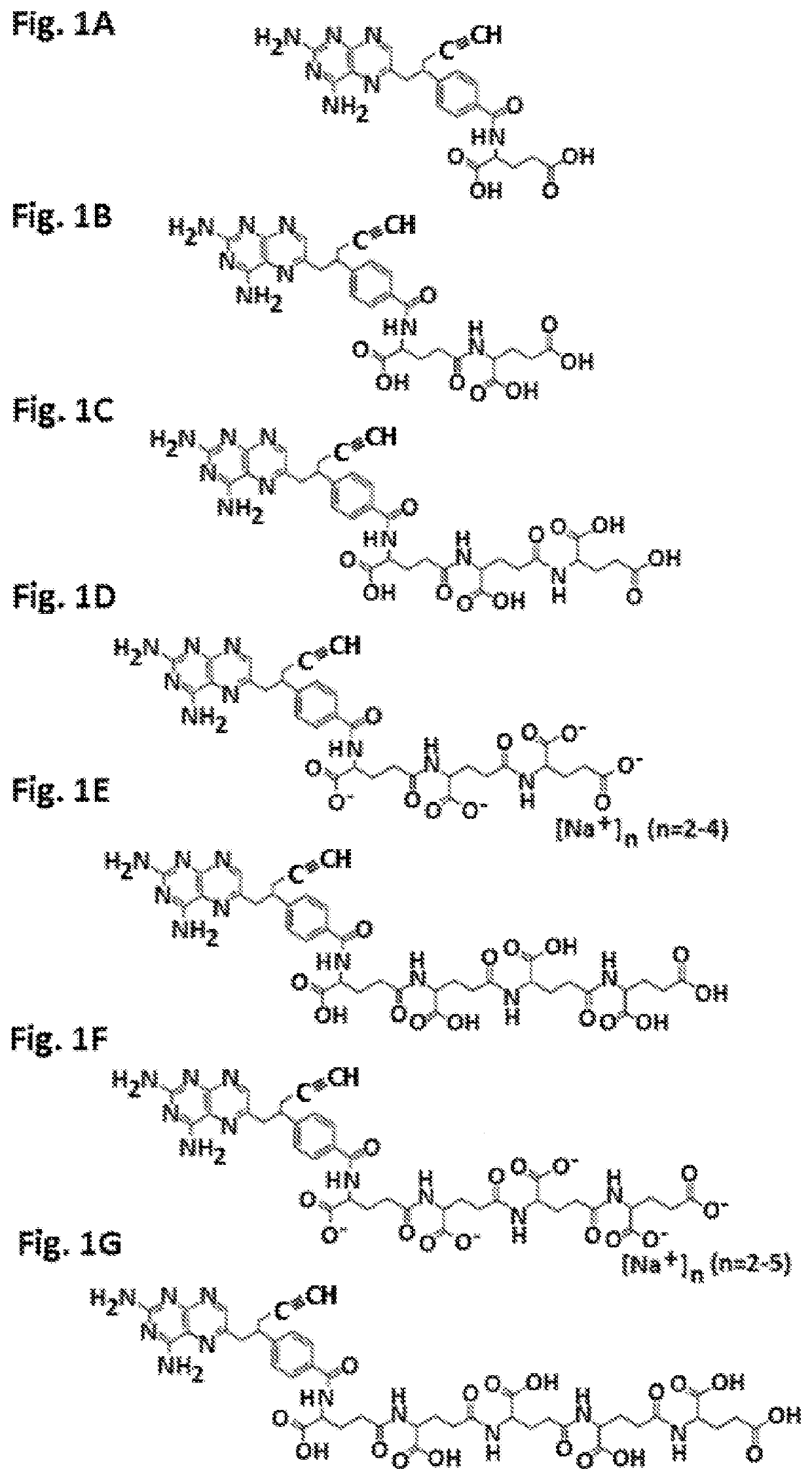
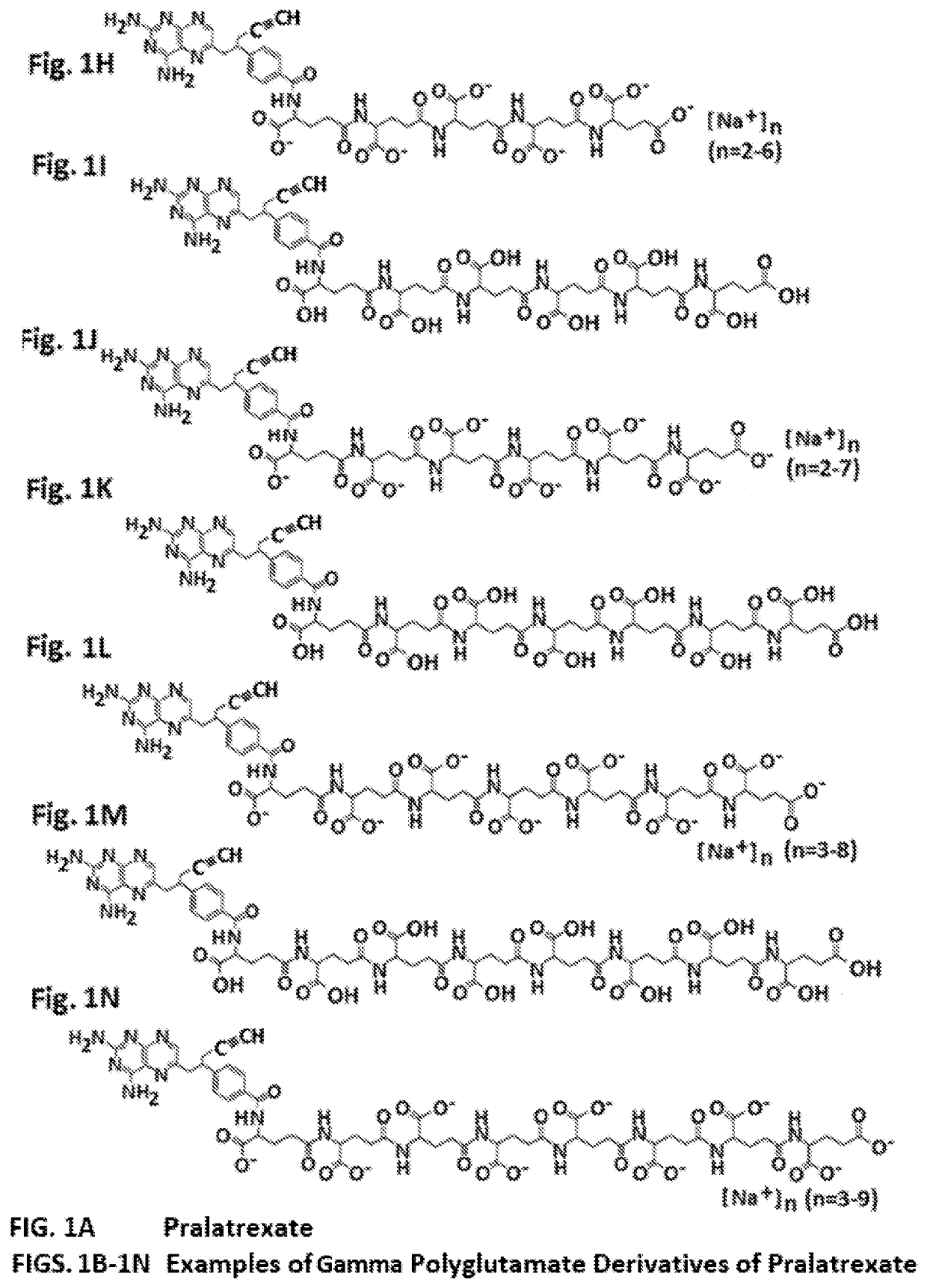
Alpha polyglutamated pralatrexate and uses thereof
PendingUS20200360390A1Improve efficacyImprove securityOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune conditionAutoimmune disease
The disclosure relates generally to alpha polyglutamated pralatrexate, formulations containing liposomes filled with alpha polyglutamated pralatrexate, methods of making the alpha polyglutamated pralatrexate and liposome containing formulations, and methods of using polyglutamated alpha polyglutamated pralatrexate and liposome containing formulations to treat hyperproliferative disorders (e.g., cancer) and disorders of the immune system (e.g., an autoimmune disease such as rheumatoid arthritis).
Owner:L E A F HLDG GRP
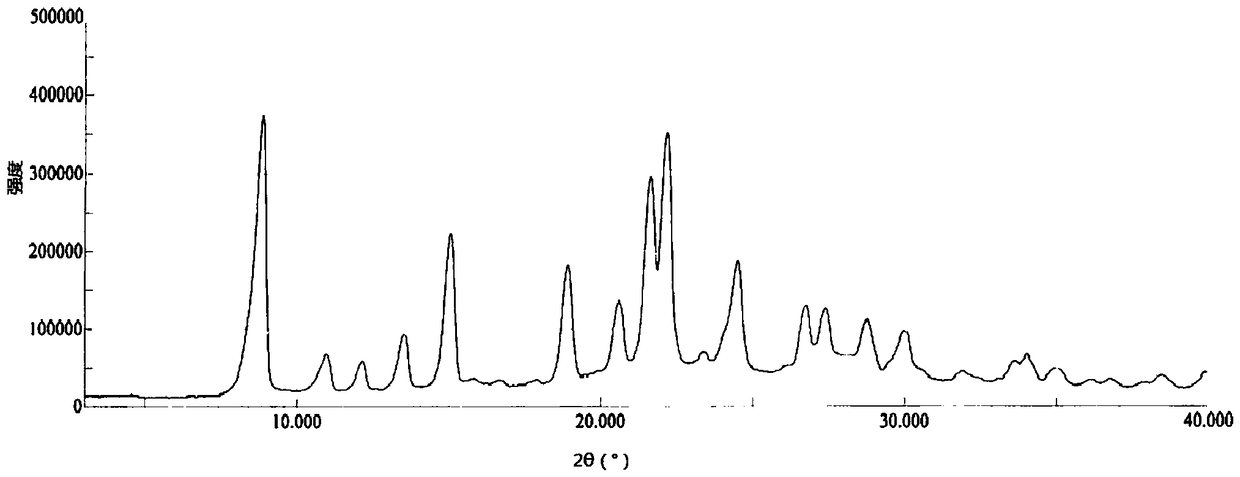
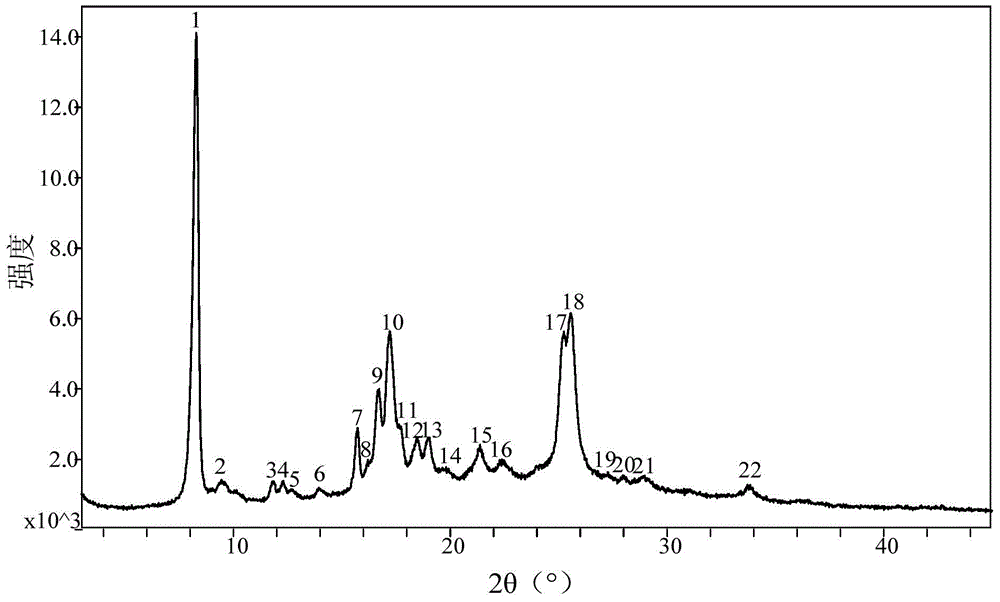
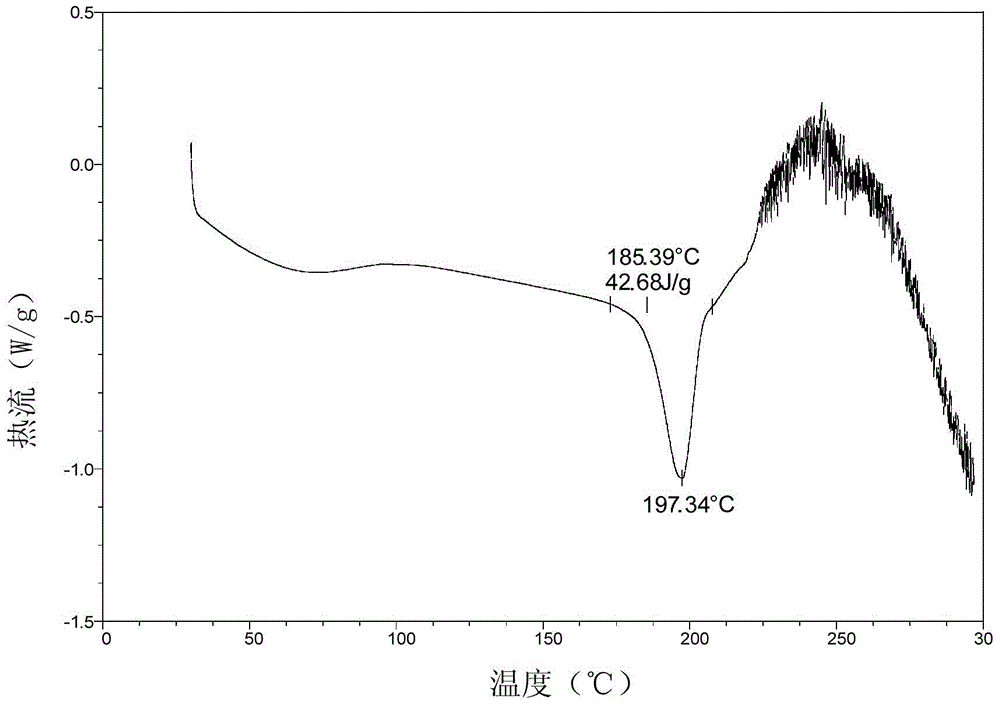
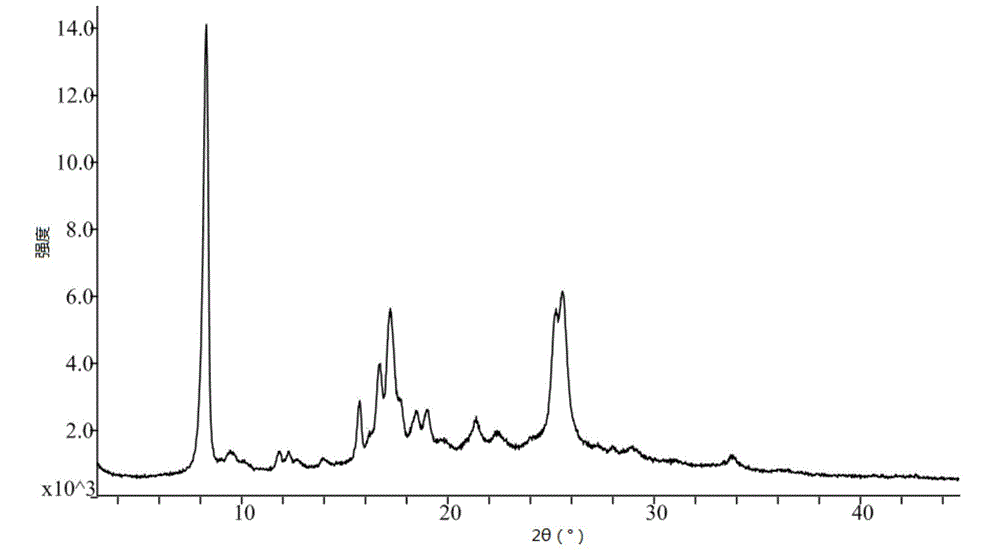
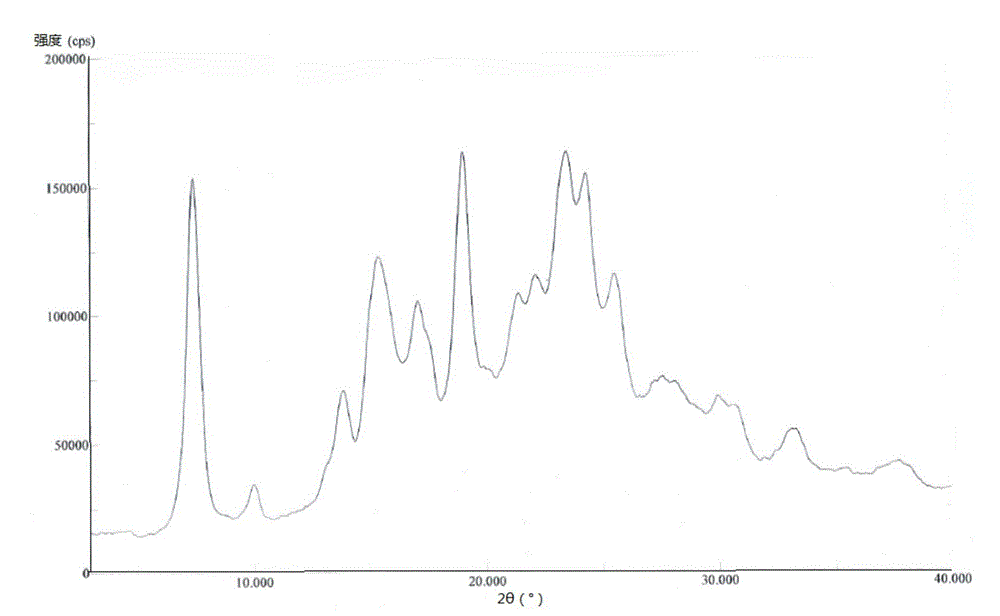
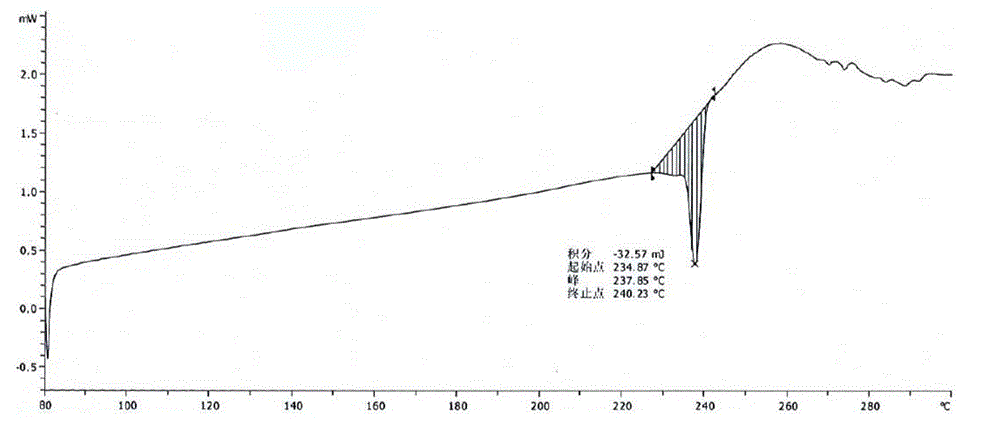
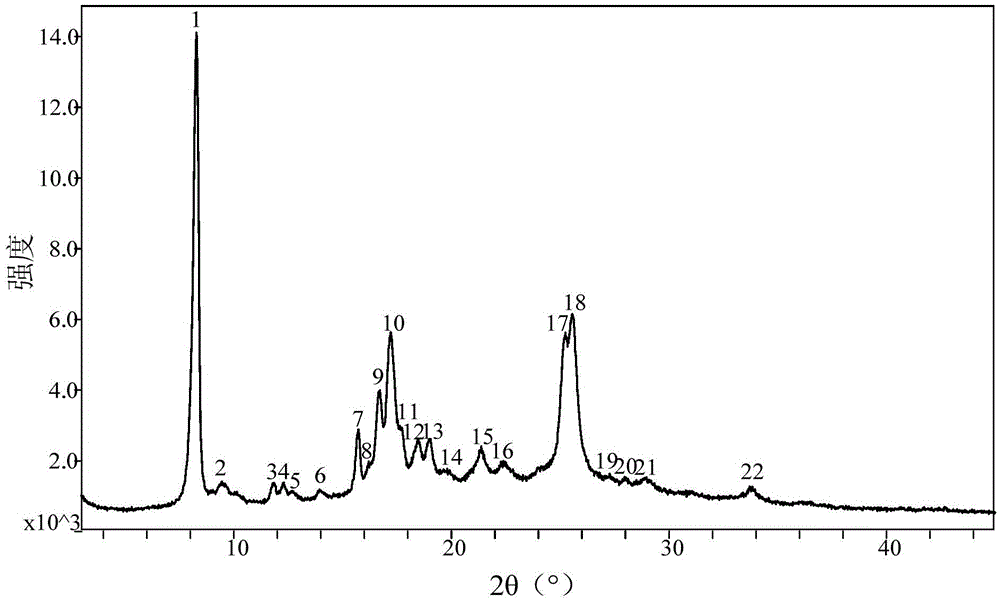
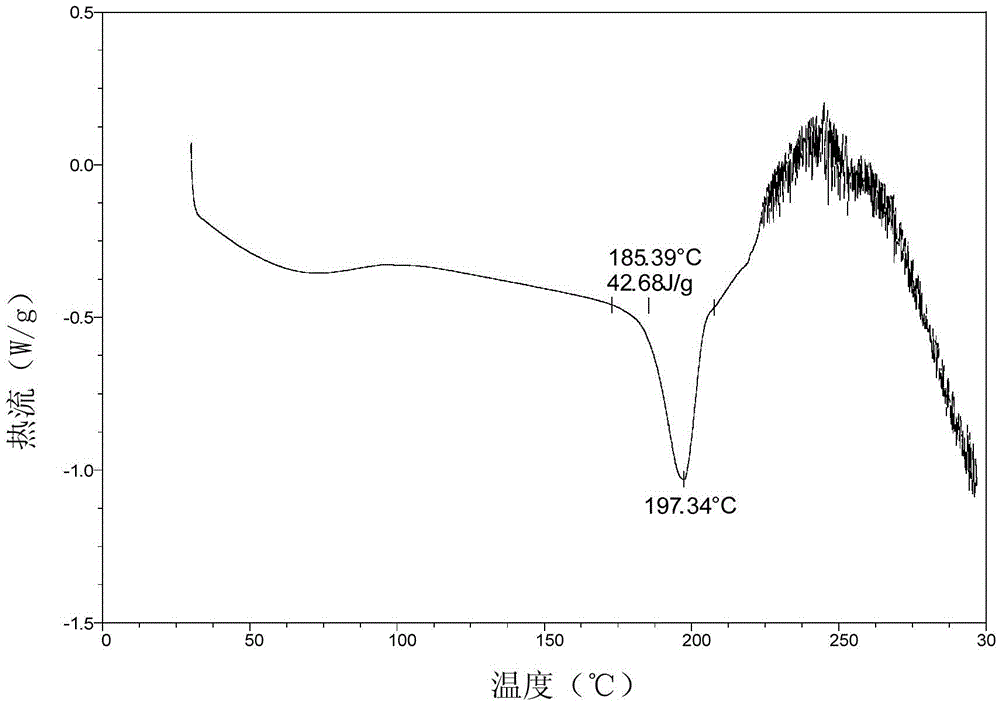
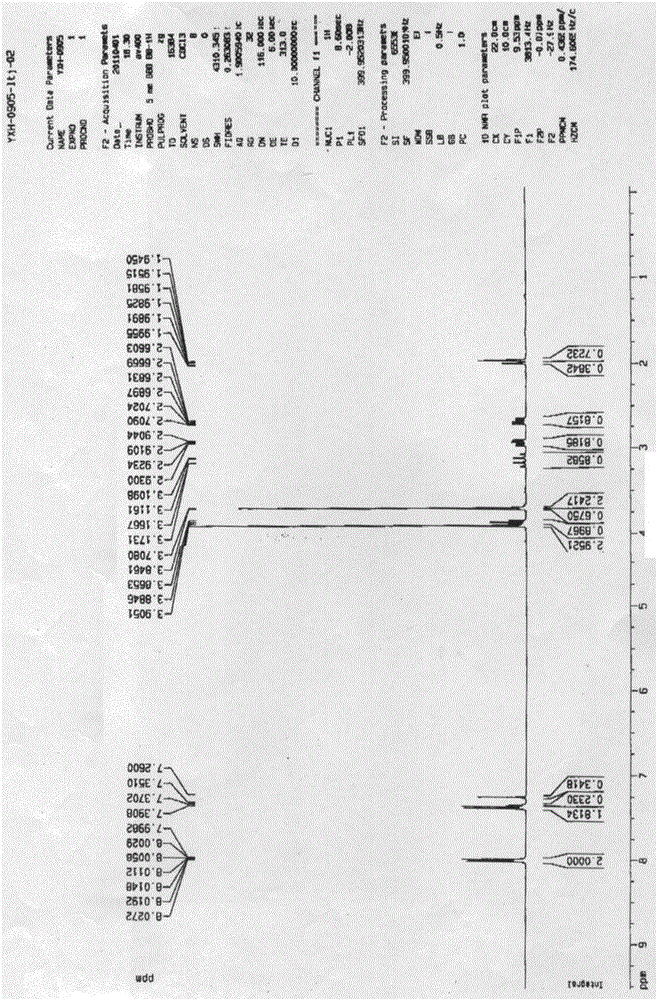
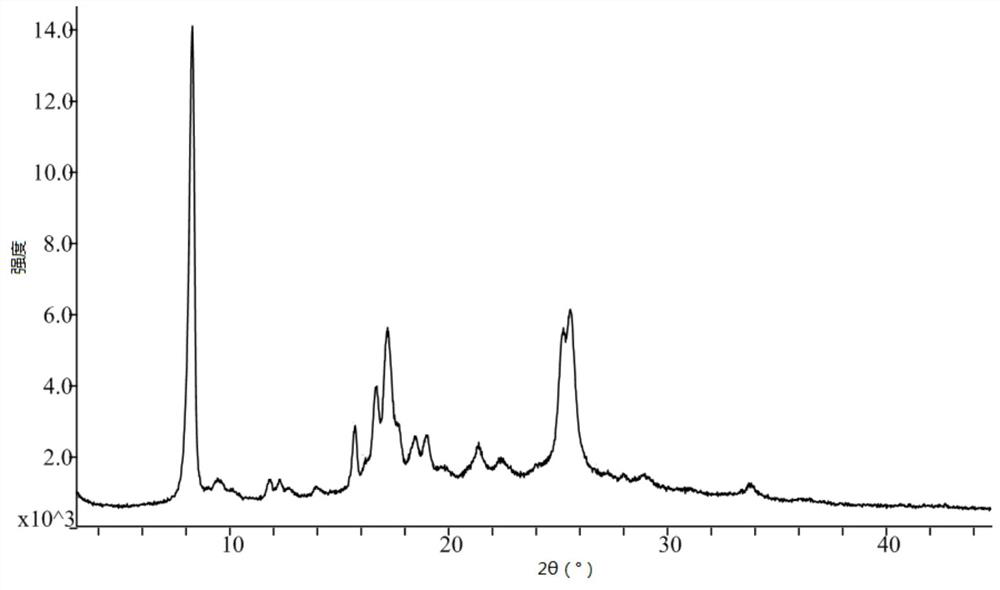
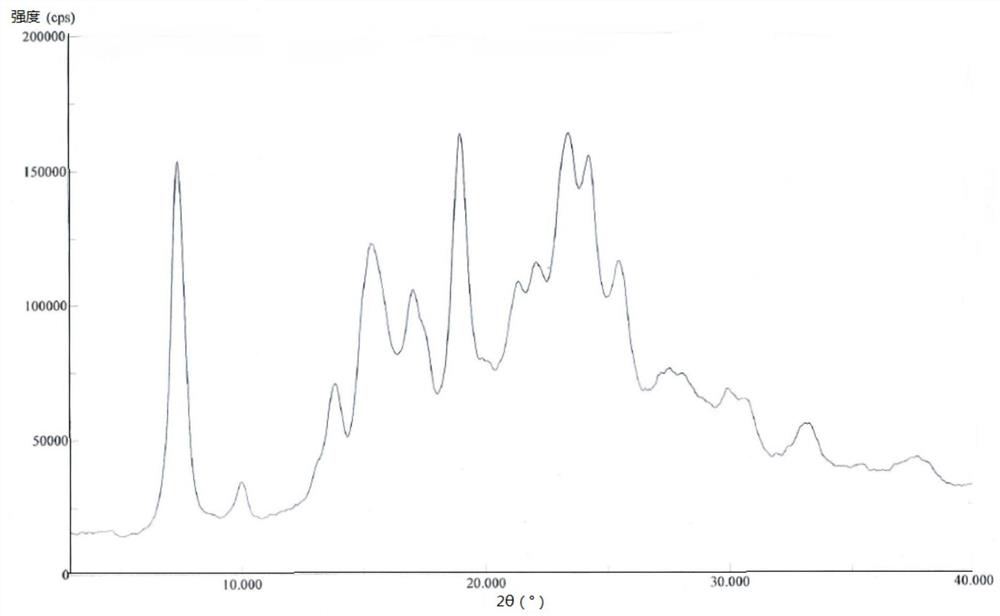
Crystal form of pralatrexate, pharmaceutical composition containing pralatrexate, and preparation method and application of pralatrexate
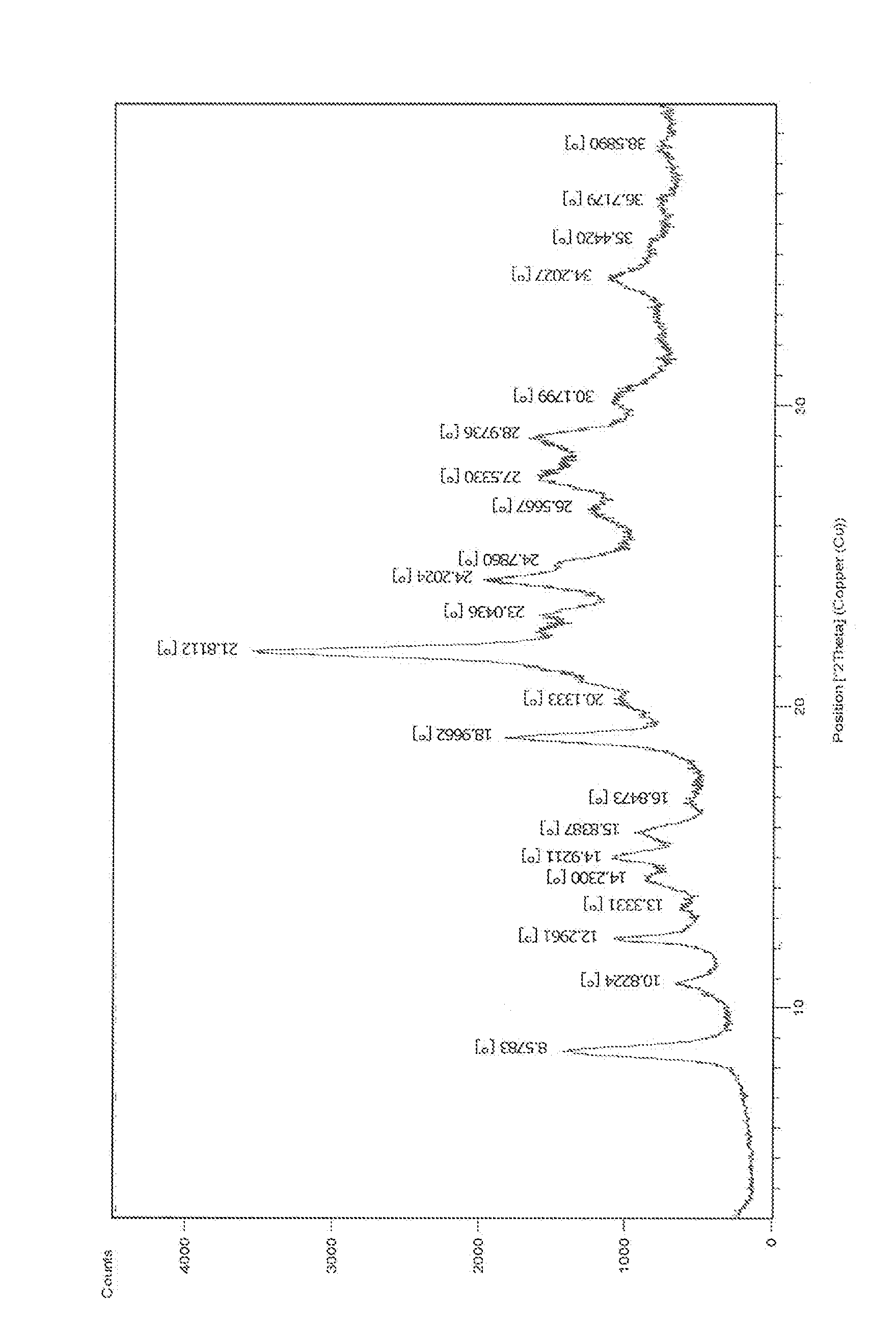
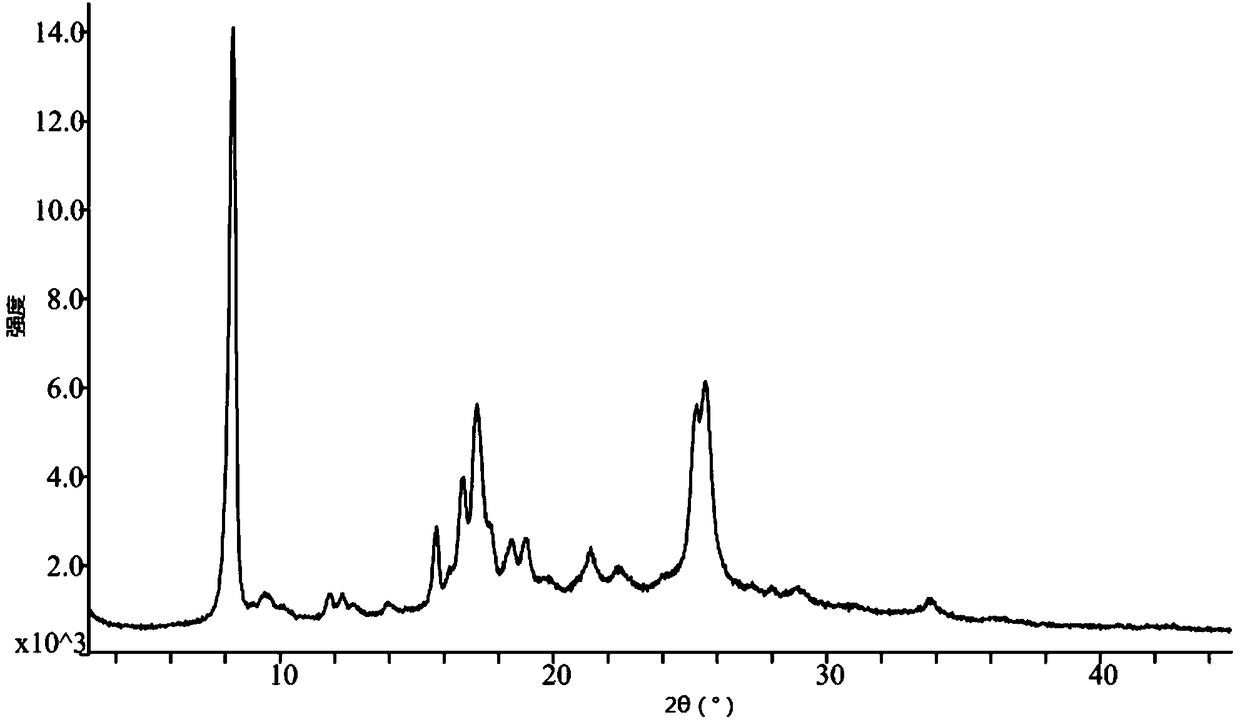
ActiveCN104418859AHigh purityImprove stabilityOrganic active ingredientsOrganic chemistryAnti solventX-ray
The invention discloses a crystal form of pralatrexate, a pharmaceutical composition containing the pralatrexate, and a preparation method and an application of the pralatrexate. According to the crystal form, in an X-ray powder diffraction pattern using a radiation source as Cu-Kalpha, characteristic absorption peaks are formed at the diffraction angles 2theta of 8.29 degrees, 15.73 degrees, 16.72 degrees, 17.21 degrees, 18.46 degrees, 19.01 degrees, 21.38 degrees, 25.25 degrees and 25.56 degrees; and the error range of 2theta is + / -0.2 degrees. The preparation method of the crystal form disclosed by the invention comprises the following steps: heating and dissolving pralatrexate into a good solvent; and dropwise adding an anti-solvent until turbidity is achieved, naturally cooling, and devitrifying, so as to obtain the crystal form. The crystal form of the pralatrexate disclosed by the invention has the advantages of high purity, good stability and the like.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
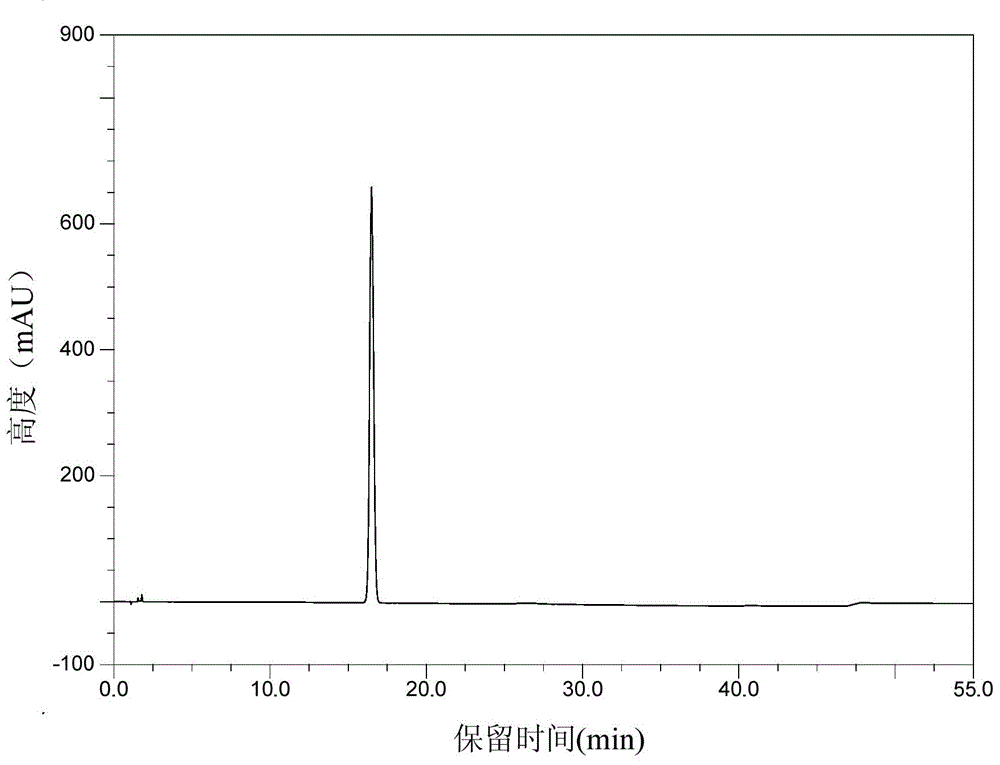
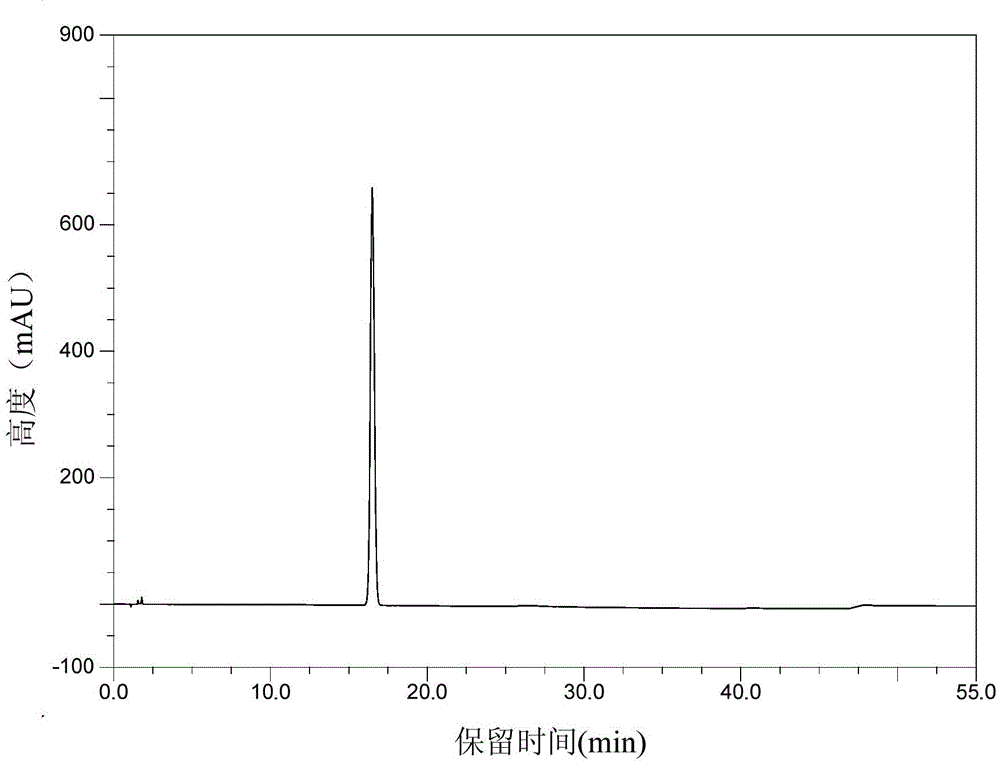
High-purity Pralatrexate solid and preparation method thereof
ActiveCN105272983AHigh purityImprove securityOrganic active ingredientsOrganic chemistryPralatrexateKetone
The invention provides a high-purity Pralatrexate solid and its preparation method. The high-purity Pralatrexate solid provided by the invention is prepared by recrystallization of an aqueous solution of lower alkyl ketone and has advantages of high purity, low individual impurity content, good stability, high safety and the like. Meanwhile, the preparation method of the high-purity Pralatrexate solid provided by the invention can be adopted to effectively remove IN0222(2,4-diamido-6-chloromethylpteridine) or its analogue and raise safety of medicine. In addition, the preparation method of the high-purity Pralatrexate solid is simple, a solvent is cheap and easily available, and crystallization condition is mild. The preparation method is suitable for industrial production.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
A kind of preparation method of pralatrexate intermediate
ActiveCN107488112BHigh selectivityHigh purityOrganic compound preparationCarboxylic acid esters preparationPhenylacetic acidPralatrexate
The invention belongs to the technical field of medicine, and in particular relates to a preparation method of a pralatrexate intermediate. The invention provides a method for preparing an intermediate α-propargyl-(4-methyl formate)-methyl phenylacetate with simpler operation and lower impurity content, especially the proportion of bispropargyl impurities generated in the product Obviously reduce, the yield of the target product α-propargyl-(4-methyl formate)-methyl phenylacetate of generation obviously increases, and the purity of product improves greatly, can be applied to large-scale production of pralatrexate bulk drug and The preparation of its intermediates.
Owner:SHANDONG NEWTIME PHARMA
Combination therapy using belinostat and pralatrexate to treat lymphoma
ActiveUS11439643B2Good effectSynergistic effectAmide active ingredientsAntineoplastic agentsCancer cellPralatrexate
The present invention relates to compositions and methods for treating lymphoma in a subject in need thereof, said methods comprising administering to the patient in need thereof a therapeutically effective amount of a combination of belinostat and pralatrexate, wherein said therapeutically effective amount results in a synergistic antiproliferative effect on cancer cell growth.
Owner:ACROTECH BIOPHARMA INC
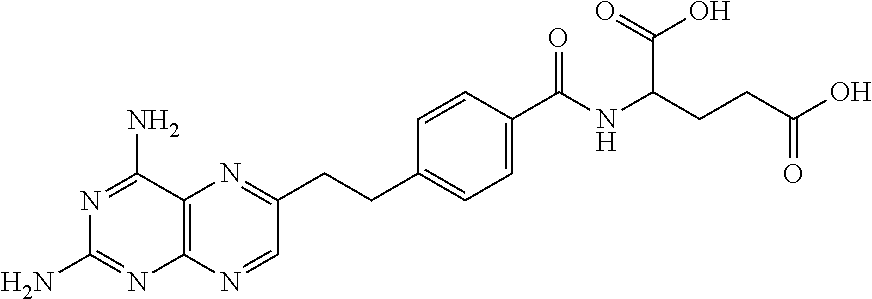
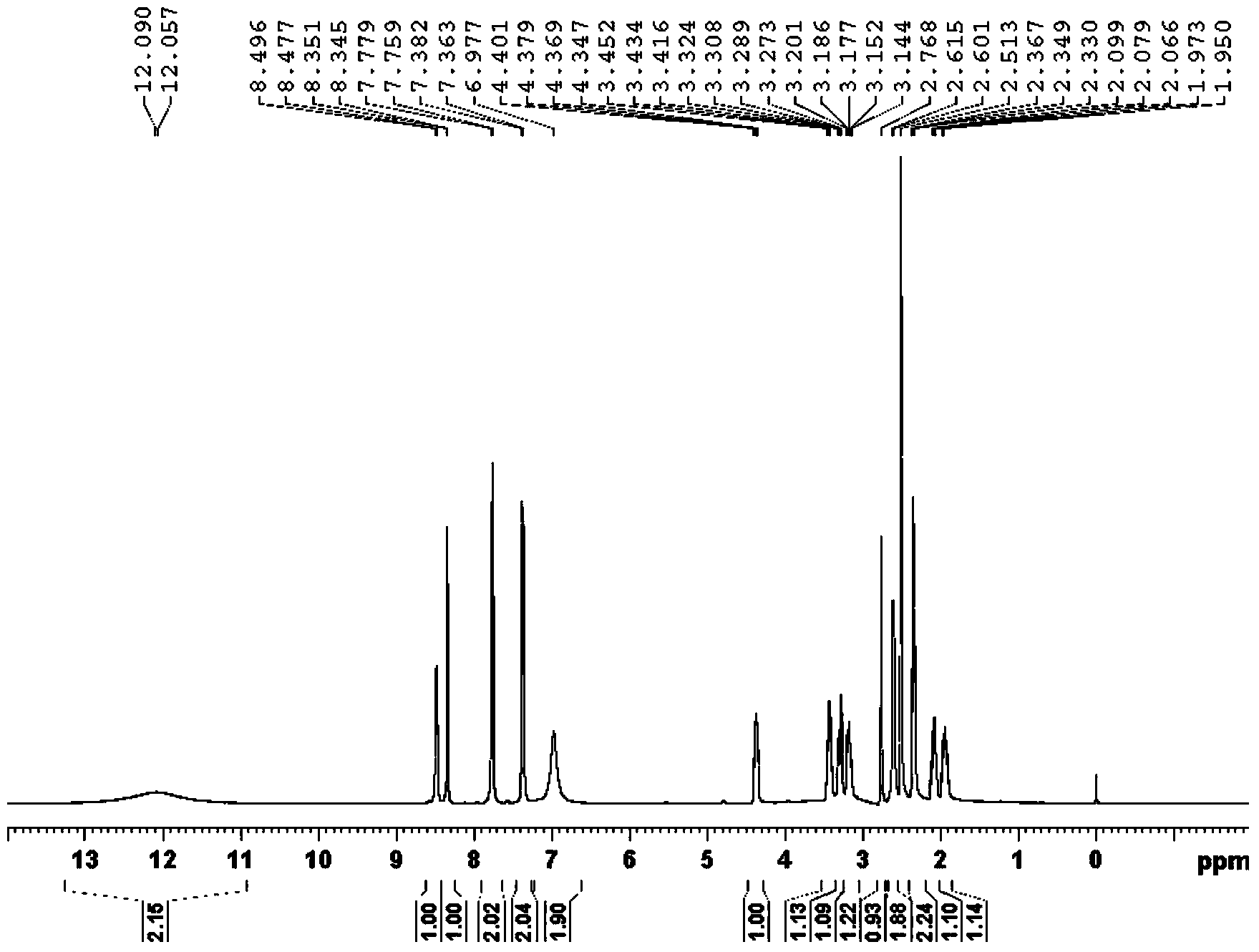
N-[4-(1-(2-propinyl)-3, 4-dioxo-n-butyl) benzoyl]-L-glutamic dialkyl ester and preparation method thereof
ActiveCN103274962BEase of industrial productionRaw materials are easy to getOrganic compound preparationCarboxylic acid amides preparationBenzoic acidPralatrexate
The invention discloses a medical intermediate N-[4-(1-(2-propinyl)-3, 4-dioxo-n-butyl) benzoyl]-L-glutamic dialkyl ester (I) and a preparation method thereof. The preparation method comprises the following steps of carrying out a condensation reaction on 4-(1-hydroxyl-3-butyne) benzoic acid (II) and L-glutamic dialkyl ester (III) to generate N-[4-(1-hydroxyl-3-butyne) benzoyl]-L-glutamic dialkyl ester (IV); and carrying out a coupled reaction on the intermediate (IV) and methylglyoxal dimethylacetal subjected to enolization to generate the N-[4-(1-(2-propinyl)-3, 4-dioxo-n-butyl) benzoyl]-L-glutamic dialkyl ester (I). According to the intermediate (I) and the preparation method thereof, a novel preparation way for the antineoplastic pralatrexate is provided and the economic and technological development of the pralatrexate is promoted.
Owner:迁安华韵知识产权服务中心
Combination therapy using belinostat and pralatrexate to treat lymphoma
ActiveUS20180177783A1Good effectSynergistic growth inhibitory effectAmide active ingredientsAntineoplastic agentsCancer cellMedicine
The present invention relates to compositions and methods for treating lymphoma in a subject in need thereof, said methods comprising administering to the patient in need thereof a therapeutically effective amount of a combination of belinostat and pralatrexate, wherein said therapeutically effective amount results in a synergistic antiproliferative effect on cancer cell growth.
Owner:ACROTECH BIOPHARMA INC
Preparation method for pralatrexate intermediate
ActiveCN107488112AHigh selectivityHigh purityOrganic compound preparationCarboxylic acid esters preparationPralatrexateFormate Esters
The invention specifically relates to a preparation method for a pralatrexate intermediate, belonging to the technical field of medicine. According to the preparation method for the intermediate alpha-propargyl-(4-methylformate)-methylphenyl acetate, operation is more convenient and the content of impurities is lower; and in particular, the ratio of dithropropyl impurities in the produced intermediate is obviously reduced, the yield of the target product alpha-propargyl-(4-methylformate)-methylphenyl acetate is significantly increased, and product purity is greatly improved. The preparation method can be applied to large-scale production of pralatrexate bulk drugs and preparation of pralatrexate intermediates.
Owner:SHANDONG NEWTIME PHARMA
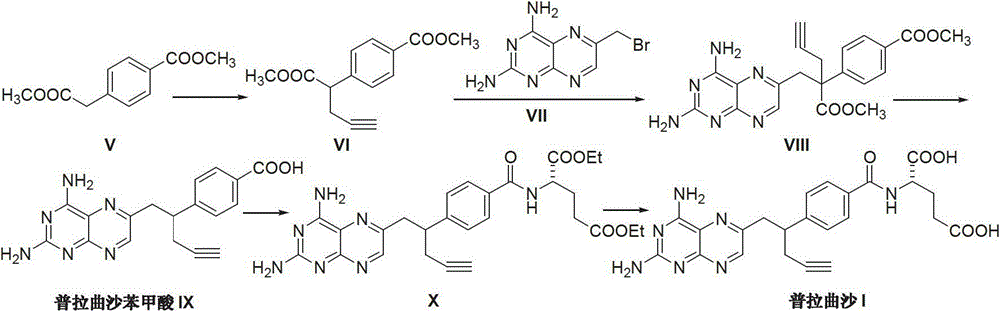
Preparation method of Pralatrexate
InactiveCN103265544BEase of industrial productionRaw materials are easy to getOrganic chemistryPralatrexateTetra
The invention discloses a preparation method of Pralatrexate (I). The preparation method comprises the following steps of: hydrolyzing N-[4-(1-(2-propinyl)-3,4-dioxo n-butyl)benzoyl]-L-glutamic acid dialkyl ester (II) to generate an intermediate N-[4-(1-(2-propinyl)-3,4-dioxo n-butyl)benzoyl]-L-glutamic acid (IV); and performign cyclization reaction on the intermediate (IV) and 2,4,5,6-tetra-aminopyrimidine (III) under the condition of oxime formation to prepare the Pralatrexate (I). The raw materials are easily available, the process is simple, and the preparation method is economical, environmentally-friendly and suitable for industrial production.
Owner:江苏兰亭市政园林有限公司
Crystal form of Pralatrexate, pharmaceutical composition containing same, preparation method and application thereof
ActiveCN104418859BHigh purityImprove stabilityOrganic active ingredientsOrganic chemistryAnti solventX-ray
The invention discloses a crystal form of pralatrexate, a pharmaceutical composition containing the pralatrexate, and a preparation method and an application of the pralatrexate. According to the crystal form, in an X-ray powder diffraction pattern using a radiation source as Cu-Kalpha, characteristic absorption peaks are formed at the diffraction angles 2theta of 8.29 degrees, 15.73 degrees, 16.72 degrees, 17.21 degrees, 18.46 degrees, 19.01 degrees, 21.38 degrees, 25.25 degrees and 25.56 degrees; and the error range of 2theta is + / -0.2 degrees. The preparation method of the crystal form disclosed by the invention comprises the following steps: heating and dissolving pralatrexate into a good solvent; and dropwise adding an anti-solvent until turbidity is achieved, naturally cooling, and devitrifying, so as to obtain the crystal form. The crystal form of the pralatrexate disclosed by the invention has the advantages of high purity, good stability and the like.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
Pralatrexate preparation method
InactiveCN103275080BEase of industrial productionRaw materials are easy to getOrganic chemistryPralatrexateHydrolysis
The invention discloses a pralatrexate (I) preparation method, which comprises the following preparation steps: performing cyclization reaction on dialkyl N-[4-(1-(2-propinyl)-3,4-dioxo-n-butyl)benzoyl]-L-glutamate (II) and 2,4,5,6-tetraminopyrimidine (III) under oximation conditions to generate an intermediate compound alkyl N-[4-[1-(2,4-diamino-6-pteridine)methyl-3-butynyl]benzoyl]-L-glutamate (IV); and performing hydrolysis reaction on the intermediate compound (IV) to prepare pralatrexate (I). The preparation method is accessible in raw materials, simple in process, economic, environment-friendly and suitable for industrial production.
Owner:安徽金太阳生化药业有限公司
Preparation method of pralatrexate
ActiveCN108069970APrevent excessive hydrolysisGuaranteed alkali concentrationOrganic chemistryTemperature controlAcetic acid
The invention belongs to the technical field of medicine and particularly relates to a preparation method of pralatrexate finished products. The method comprises the following steps: by taking PLQS-6as a raw material and respectively taking acetone, methyl ethyl ketone, butanone, dioxane and the like as solvents, performing temperature control, and dropwise adding inorganic base in batches to control the generation of impurities; after the reaction is completed, dropwise adding a crystallization solvent to enable pralatrexate to be crystallized; collecting pralatrexate crystallized solids; adding the solids to purified water to be dissolved; performing washing with dichloromethane; and then, adjusting the pH value with acetic acid to obtain pralatrexate solids. The synthetic method provided by the invention is simple and easy to operate, the impurities are easy to remove, the yield and the purity of the prepared pralatrexate are higher, and the method is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of refining method of pralatrexate intermediate
ActiveCN108069971BHigh purityEasy to recycleOrganic chemistryChromatographic separationActivated carbon
The invention belongs to the technical field of medicine, and in particular relates to a method for refining a pralatrexate intermediate. The method comprises the steps of: adding the crude product of the intermediate 10-propargyl-10-desazaaminopterin dimethyl ester (PLQS-6) into a refined solvent, heating and dissolving, decolorizing activated carbon, hot filtering, cooling the filtrate and stirring for crystallization, The filter cake is washed with a refined solvent to obtain a high-purity pralatrexate intermediate PLQS‑6. The refined solvents used are all low-boiling solvents, which not only avoids the problem of excessive dissolved residues of DMF and DMSO, but also facilitates the recycling of refined solvents and saves costs. The application of column chromatographic separation is avoided, the yield is improved, the entire process operation is simplified, the operation method is simple, the yield and purity are high, and the method is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
A preparation method suitable for industrial scale-up production of pralatrexate
ActiveCN103739604BEasy to operateQuality is easy to controlOrganic chemistryBiochemical engineeringPralatrexate
The invention relates to the technical field of medicinal chemistry, in particular to the synthesis of anticancer drug 10-Propargyl-10-deazaaminopterin Pralatrexate and its intermediates. The present invention provides a method with simpler operation, lower product-related impurity content, easier purification of intermediates, avoiding the purification method of column chromatography, and can be applied to the preparation of large-scale production of pralatrexate raw materials and its important intermediates and purification methods.
Owner:SINOPHARM A THINK PHARMA
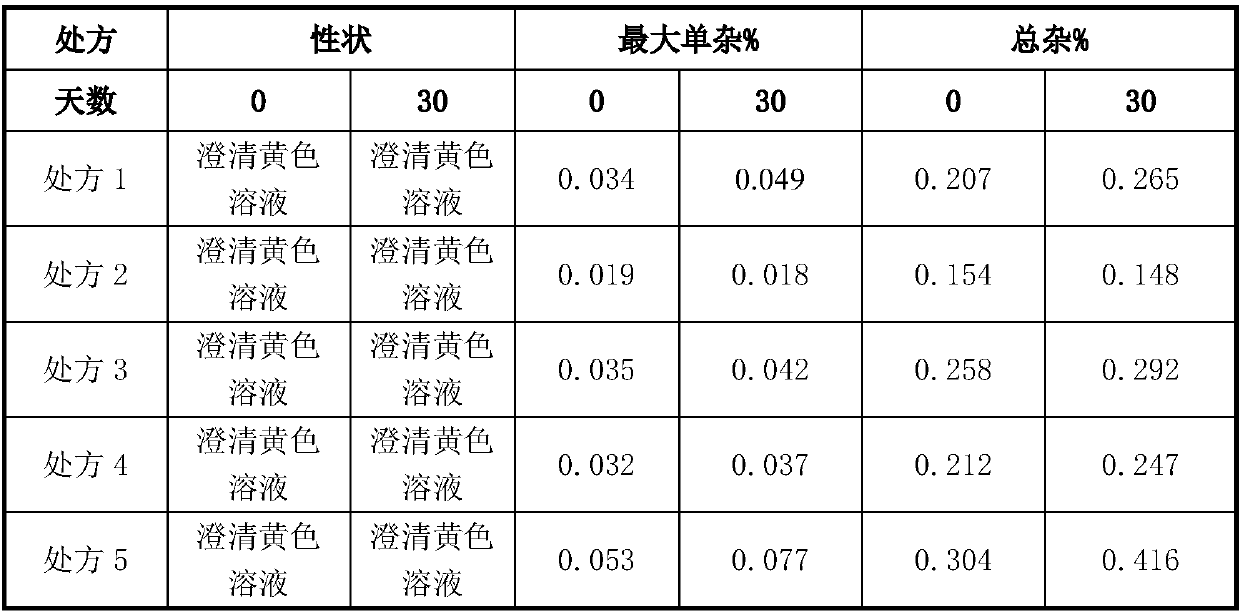
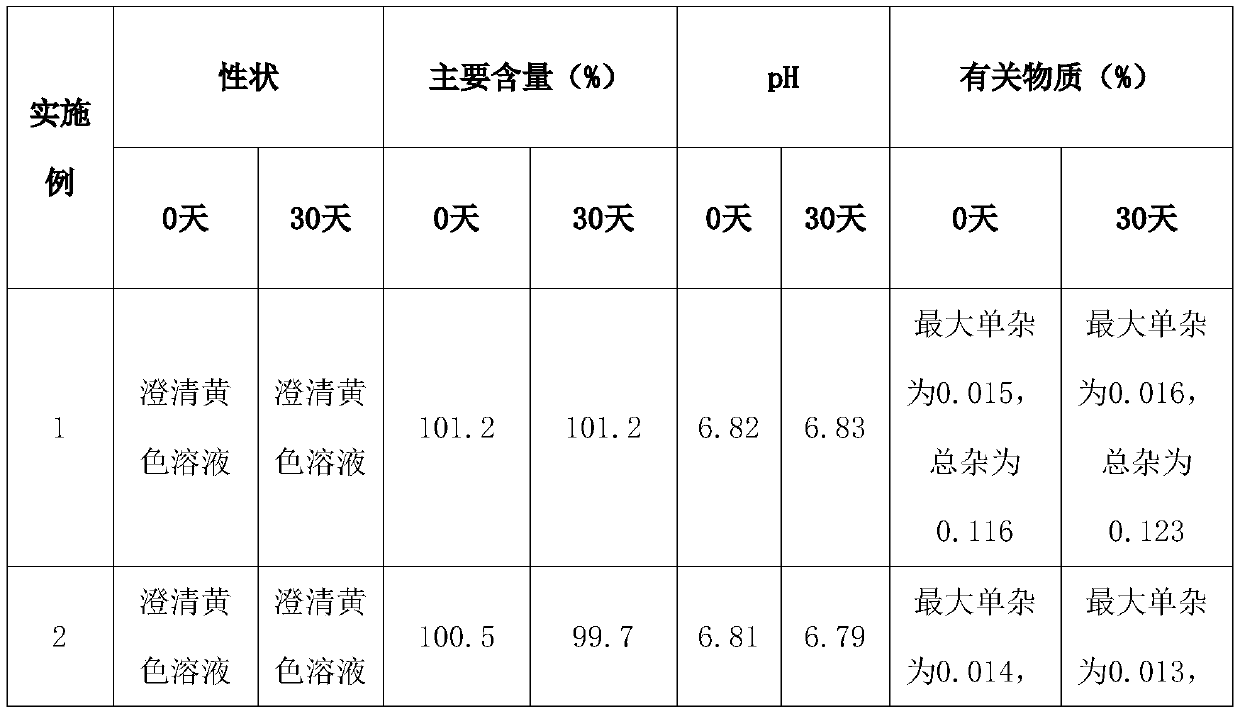
Pralatrexate injection as well as preparation method and application thereof
InactiveCN111557905ASimple processReduce manufacturing costOrganic active ingredientsInorganic non-active ingredientsPralatrexateMedicinal chemistry
The invention relates to a pralatrexate composition, and especially relates to a stable pralatrexate injection as well as a preparation method and application thereof. The pralatrexate injection comprises pralatrexate, a pH regulator, an isotonic agent and water for injection, and optionally further comprises a stabilizer, wherein the pH value of the pralatrexate injection is 6.5-7.3. The pralatrexate injection prepared by the invention has the advantages of simple process, low cost, safety, reliability, good stability, convenience in clinical use, suitability for industrial production and thelike.
Owner:JIANGSU HANSOH PHARMA CO LTD
A kind of high-purity pralatrexate solid and preparation method thereof
ActiveCN105272983BHigh purityImprove securityOrganic active ingredientsOrganic chemistryPharmaceutical drugPralatrexate
The invention provides a high-purity Pralatrexate solid and its preparation method. The high-purity Pralatrexate solid provided by the invention is prepared by recrystallization of an aqueous solution of lower alkyl ketone and has advantages of high purity, low individual impurity content, good stability, high safety and the like. Meanwhile, the preparation method of the high-purity Pralatrexate solid provided by the invention can be adopted to effectively remove IN0222(2,4-diamido-6-chloromethylpteridine) or its analogue and raise safety of medicine. In addition, the preparation method of the high-purity Pralatrexate solid is simple, a solvent is cheap and easily available, and crystallization condition is mild. The preparation method is suitable for industrial production.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Gamma polyglutamated pralatrexate and uses thereof
PendingUS20210346294A1Eliminate side effectsOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune conditionDelivery vehicle
The disclosure relates generally to gamma polyglutamated pralatrexate compositions, including delivery vehicles such as liposomes containing the gamma polyglutamated pralatrexate, and methods of making and using the gamma polyglutamated pralatrexate compositions to treat hyperproliferative disorders (e.g., cancer) and disorders of the immune system (e.g., inflammation and autoimmune diseases such as rheumatoid arthritis).
Owner:L E A F HLDG GRP
4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof
ActiveCN103274943BEasy to manufactureImprove economyOrganic compound preparationCarboxylic acid esters preparationBenzaldehydeMetal catalyst
The invention discloses a novel medical compound 4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate (I) and a preparation method thereof. The preparation method comprises the following steps of carrying out an addition reaction on 4-carbalkoxy benzaldehyde (II) and 3-propargyl bromide (III) to generate 4-(1-hydroxyl-3-butyne) benzoate (IV); and carrying out a coupled reaction on an intermediate (IV) and pyruvaldehyde dialkyl acetal (V) after being subjected to enolization under the action of a metal catalyst to form the 4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate (I). According to the intermediate (I) and the preparation method thereof, a novel preparation way for the antineoplastic pralatrexate is provided and the economic and technological development of the pralatrexate is promoted.
Owner:临沂经开财金投资发展有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof 4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof](https://images-eureka.patsnap.com/patent_img/fd4ad065-e481-4d91-bfa4-fa6d020df7a1/FDA00003240551000011.png)
![4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof 4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof](https://images-eureka.patsnap.com/patent_img/fd4ad065-e481-4d91-bfa4-fa6d020df7a1/BDA00003240551100011.png)
![4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof 4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof](https://images-eureka.patsnap.com/patent_img/fd4ad065-e481-4d91-bfa4-fa6d020df7a1/BDA00003240551100021.png)
![N-[4-(1-(2-propinyl)-3, 4-dioxo-n-butyl) benzoyl]-L-glutamic dialkyl ester and preparation method thereof N-[4-(1-(2-propinyl)-3, 4-dioxo-n-butyl) benzoyl]-L-glutamic dialkyl ester and preparation method thereof](https://images-eureka.patsnap.com/patent_img/594b1bba-8436-48c4-a0e0-7bbdeae6b92a/FDA0000622573580000011.PNG)
![N-[4-(1-(2-propinyl)-3, 4-dioxo-n-butyl) benzoyl]-L-glutamic dialkyl ester and preparation method thereof N-[4-(1-(2-propinyl)-3, 4-dioxo-n-butyl) benzoyl]-L-glutamic dialkyl ester and preparation method thereof](https://images-eureka.patsnap.com/patent_img/594b1bba-8436-48c4-a0e0-7bbdeae6b92a/GDA0000622573590000011.PNG)
![N-[4-(1-(2-propinyl)-3, 4-dioxo-n-butyl) benzoyl]-L-glutamic dialkyl ester and preparation method thereof N-[4-(1-(2-propinyl)-3, 4-dioxo-n-butyl) benzoyl]-L-glutamic dialkyl ester and preparation method thereof](https://images-eureka.patsnap.com/patent_img/594b1bba-8436-48c4-a0e0-7bbdeae6b92a/GDA0000622573590000021.PNG)

![4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof 4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof](https://images-eureka.patsnap.com/patent_img/efabff1b-420b-49a4-9fe7-86a549126cf2/BDA00003240551100011.PNG)
![4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof 4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof](https://images-eureka.patsnap.com/patent_img/efabff1b-420b-49a4-9fe7-86a549126cf2/BDA00003240551100021.PNG)
![4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof 4-[1-(2-propinyl)-3, 4-dioxo-n-butyl] benzoate and preparation method thereof](https://images-eureka.patsnap.com/patent_img/efabff1b-420b-49a4-9fe7-86a549126cf2/BDA00003240551100022.PNG)