Crystal form of pralatrexate, pharmaceutical composition containing pralatrexate, and preparation method and application of pralatrexate
A technology of crystal forms and drugs, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, can solve the problems of poor stability and low purity of crystal forms, and achieve good stability, simple preparation methods, and easy solvents. The effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] Pratroxa preparation method: according to DeGraw et al. in the literature "Synthesis and Antitumor Activity of 10-Propargyl-10-deazaaminopterin" J. Med. Chem. 36: 2228-2231 (1993) provided the preparation method of the preparation method.
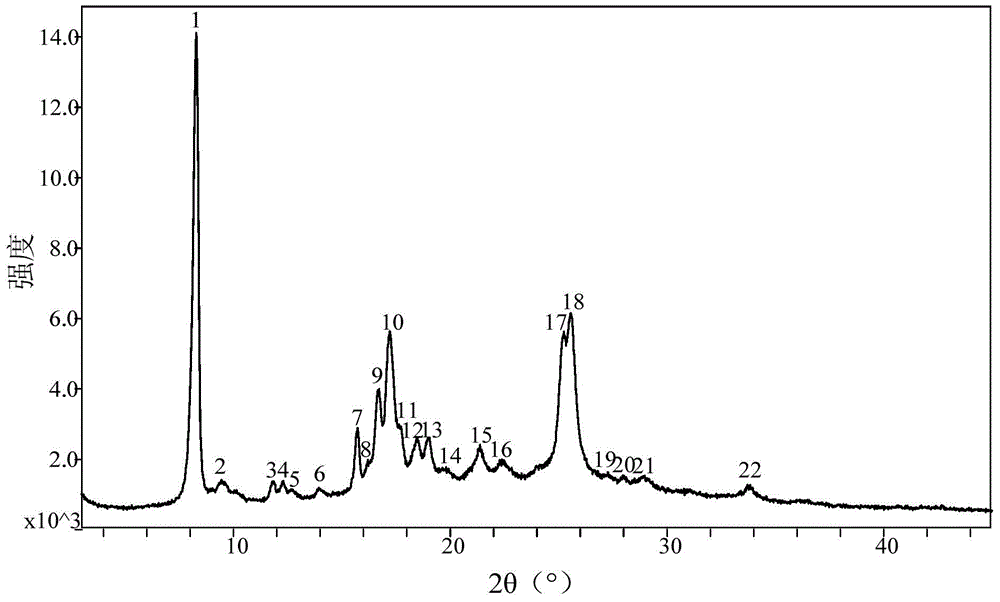
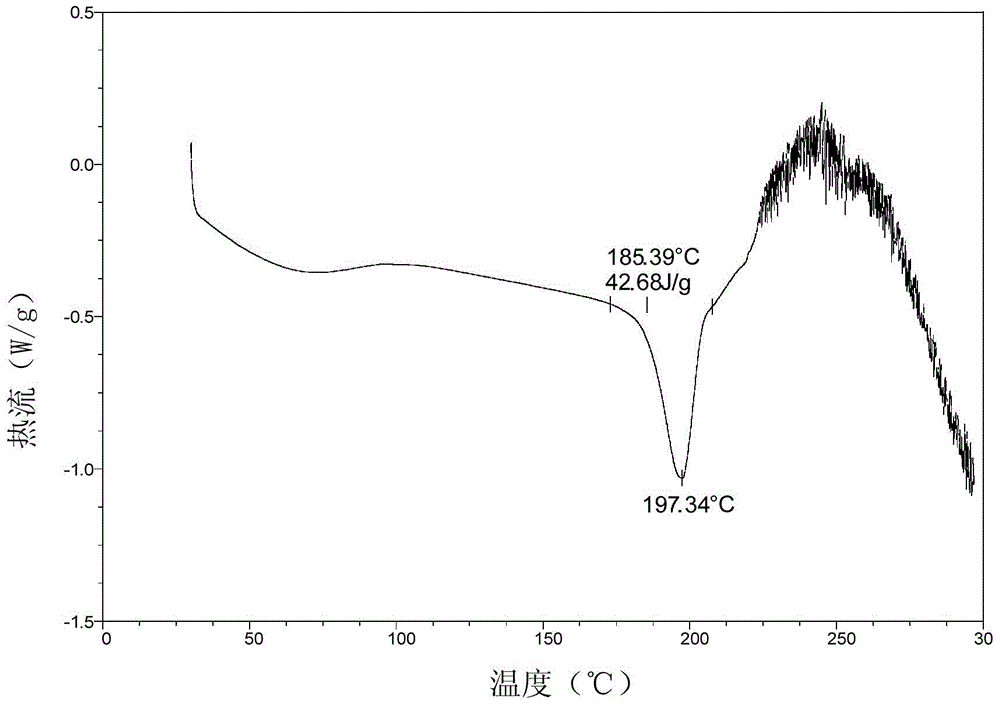
[0029] The preparation method of the crystalline form of Pratroxa: heating and dissolving Pratroxa in a good solvent, then adding anti-solvent until turbidity appears, cooling and crystallization.
Embodiment 1
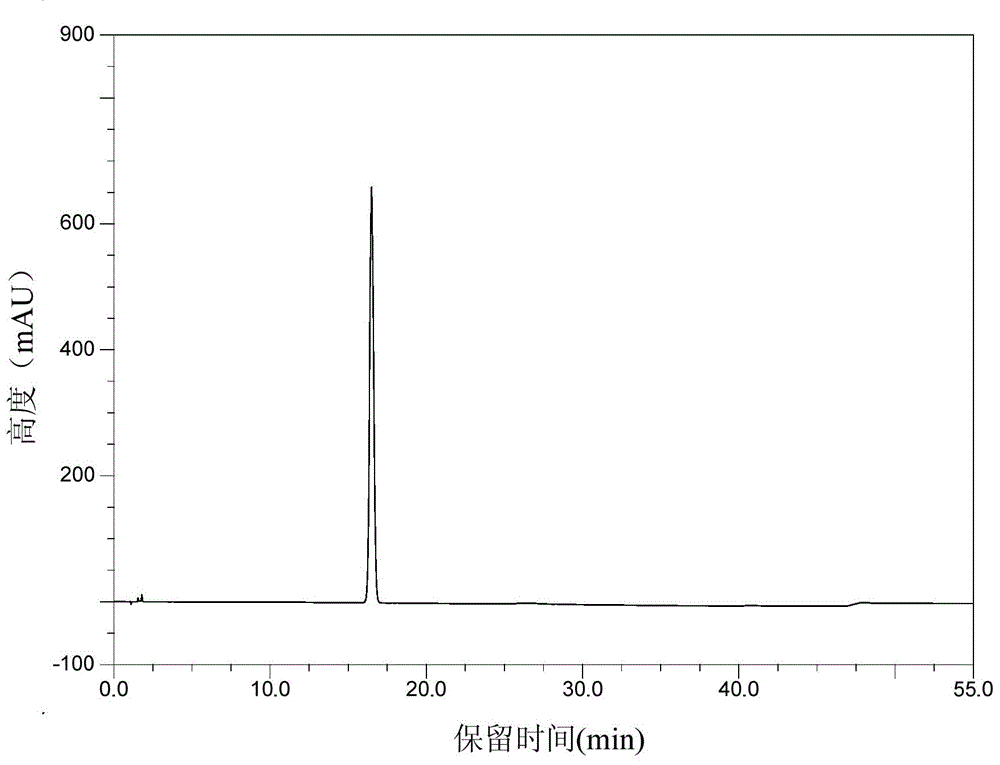
[0033] Take 15.0g of Pratroxa (purity 98.01%) and add 100ml N,N-dimethylformamide, heat to 50℃, stir for 10 minutes, add 50ml acetonitrile dropwise, cool naturally to crystallize, and keep at room temperature 25℃ for 4 hours Continue to crystallize, filter, rinse the filter cake with 30 ml of acetonitrile, and dry the filter cake in vacuum at 50° C. to obtain 12.1 g of the crystal form of Pratroxa (purity 99.68%).
Embodiment 2
[0035] Take 15.0g of Pratroxa (purity 97.91%) and add 50ml of N,N-dimethylformamide, raise the temperature to 80°C, stir for 10 minutes, add 25ml of acetonitrile dropwise, cool naturally to crystallize, and keep at room temperature 25°C for 4 hours Continue to crystallize, filter, rinse the filter cake with 30 ml of acetonitrile, and dry the filter cake in vacuum at 50° C. to obtain 13.3 g of the crystal form of Pratroxa (purity: 99.52%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com