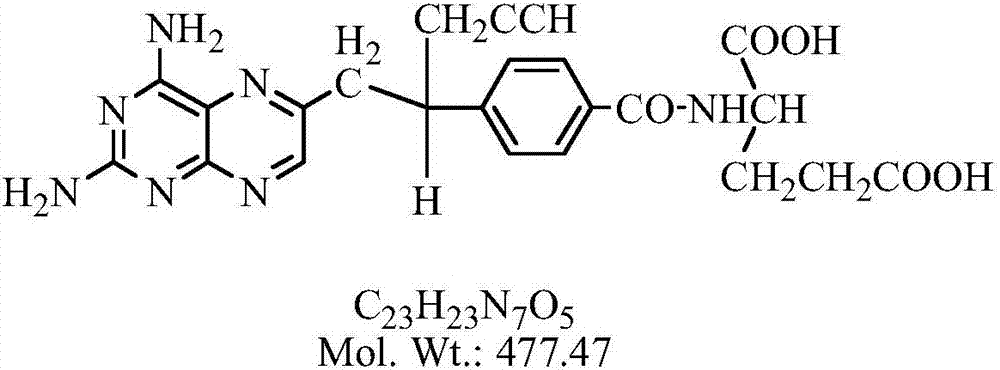
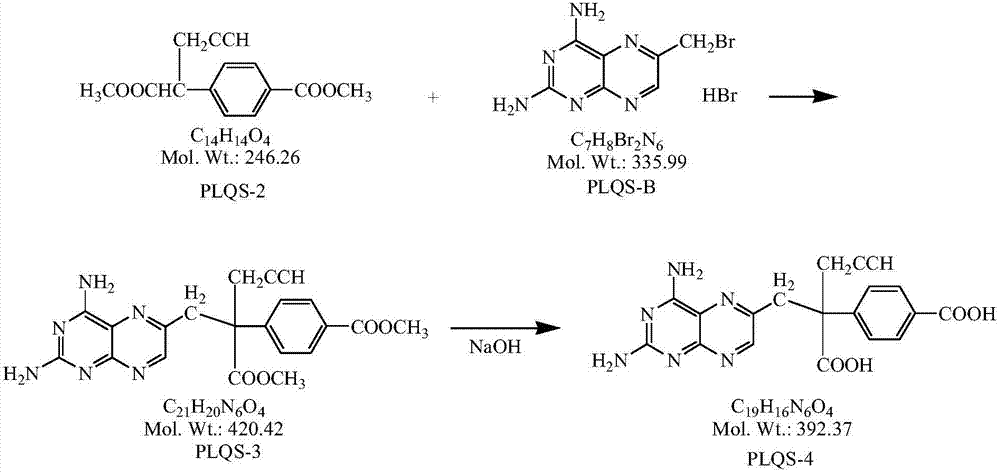
Preparation method for high-purity pralatrexate intermediate
A technology for pralatrexate and an intermediate, which is applied in the field of medicine, can solve the problems of unsuitable filtration, long refining time, low purity and the like, and achieve the effects of shortening post-processing time, improving work efficiency and reducing solid viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Under the protection of inert gas, add 400ml DMF and 32.5g NaH into a 1L three-necked flask, and when the internal temperature drops to -5~0°C, start to add the anhydrous DMF solution of PLQS-2 dropwise (dissolve 50g PLQS-2 in 65ml in anhydrous DMF), the rate of addition was controlled so that the internal temperature was not higher than 0°C. After dropping, continue to stir at 0-5°C for 1 hour, lower the temperature, and when the internal temperature drops to -20--10°C, slowly add 600ml of PLQS-B anhydrous DMF solution dropwise, and control the rate of addition so that the internal temperature is not higher than - 10°C (about 2 to 3 hours to drop). After dripping, continue to insulate and stir for 0.5h. After the reaction is completed, pour the reaction solution into 5L of anhydrous ether. Solids are precipitated, continue to stir for 0.5h, and filter with suction. Add 2.0L of anhydrous methanol to the filter cake and heat to reflux for 2h. Cool down to room temperatu...
Embodiment 2
[0030] Under the protection of inert gas, add 400ml DMF and 32.5g NaH into a 1L three-necked flask, and when the internal temperature drops to -5~0°C, start to add the anhydrous DMF solution of PLQS-2 dropwise (dissolve 50g PLQS-2 in 65ml in anhydrous DMF), the rate of addition was controlled so that the internal temperature was not higher than 0°C. After dropping, continue to stir at 0-5°C for 1 hour, lower the temperature, and when the internal temperature drops to -20--10°C, slowly add 600ml of PLQS-B anhydrous DMF solution dropwise, and control the rate of addition so that the internal temperature is not higher than - 10°C (about 2 to 3 hours to drop). After dripping, continue to keep warm and stir for 0.5h. After the reaction is completed, pour the reaction solution into 5L of methyl tert-butyl ether. Solids are precipitated, continue to stir for 0.5h, filter with suction, add 2.0L of absolute ethanol to the filter cake and heat to reflux After 2 hours, cool down to room...
Embodiment 3
[0032] Add 65g of PLQS-3 and 650ml of purified water into a 3L three-necked flask, cool down to about 10°C, add 1.55L of 1mol / L sodium hydroxide aqueous solution dropwise under stirring, and continue the heat preservation reaction for 24-30h after the dropwise addition, sample HPLC, and react After completion, add concentrated hydrochloric acid dropwise to the reaction liquid at room temperature until the pH value is 3.5, solids precipitate out, stir for 2 hours, filter, add 10 times absolute ethanol to the filter cake and heat to reflux for 1 hour, filter out insolubles, and the filtrate naturally cools down and stirs to analyze Crystallization, when the solid precipitates, continue to stir and crystallize in an ice bath for 2h, filter with suction, wash the filter cake with 50ml of absolute ethanol, filter and dry it and put it in a 50°C oven to dry to constant weight to obtain 60.65g of a brownish yellow solid with a purity of : 98.7%, yield 84.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com