Method for preparing rare earth fluoride
A technology of rare earth fluoride and rare earth oxide, which is applied in chemical instruments and methods, rare earth metal compounds, inorganic chemistry, etc., can solve the problems of hydrogen fluoride gas protection and tail gas treatment difficulties, increase of non-rare earth impurities, complex equipment, etc., to facilitate industrialization Production, no corrosion of equipment, less energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
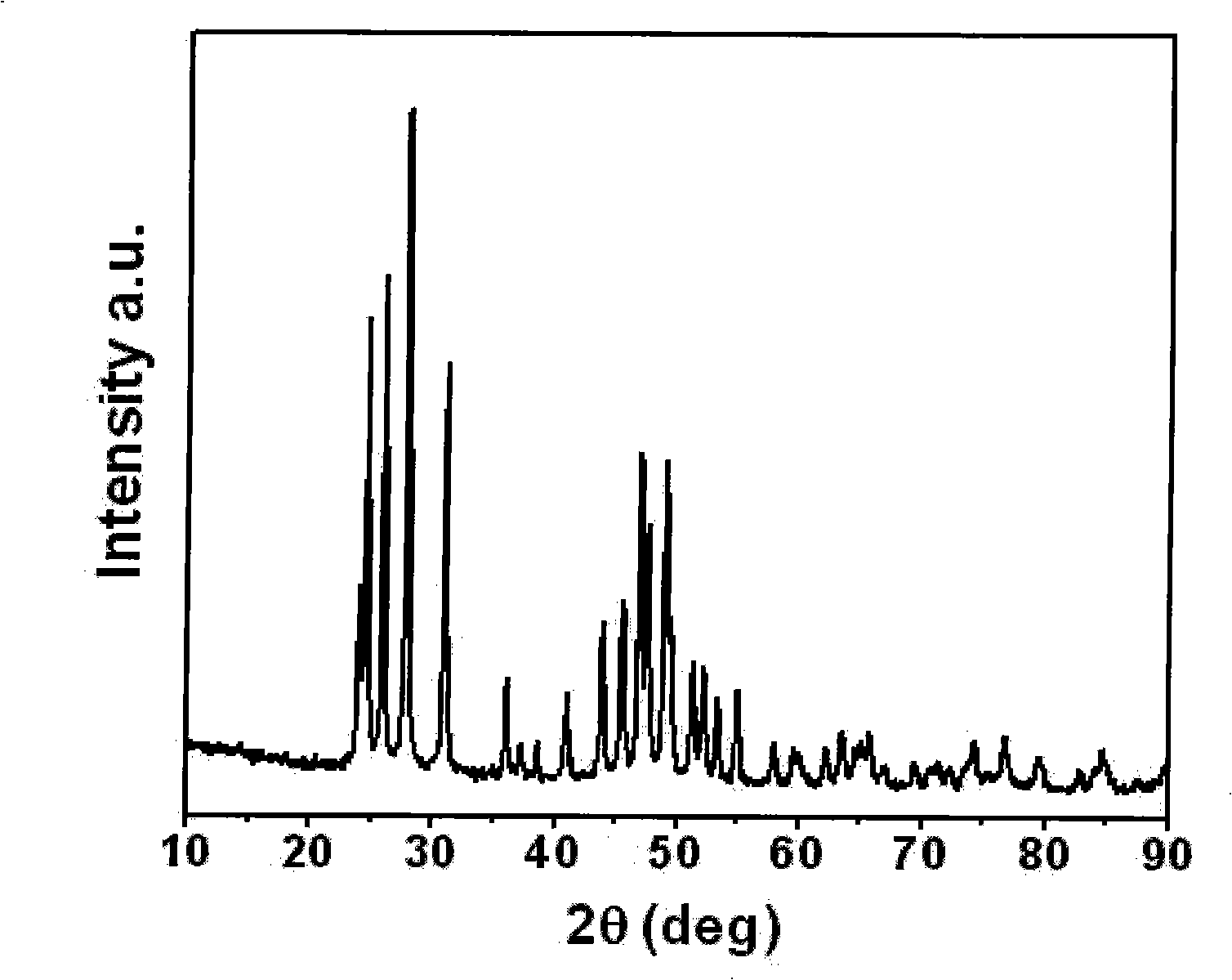
[0022] A specific preparation method of the present invention is: Yttrium oxide (Y 2 o 3 ) dissolved in 30% dilute acetic acid, Y 2 o 3 The molar ratio with acetic acid is 1:6 to form transparent yttrium acetate aqueous solution; ammonium fluoride (NH 4 F) dissolved in deionized water, NH 4 The dosage of F is Y 2 o 3 6 times the molar amount to form NH 4 F transparent aqueous solution; the aqueous solution of yttrium acetate and NH 4 The F aqueous solution was mixed and reacted to form a slurry liquid, which was ultrasonically mixed for 0.5 hours with an ultrasonic cleaner, and then yttrium fluoride (YF 3 ); the precipitated YF 3 Put it in a vacuum drying oven, dry it at 70°C, and then sinter it in the air at 300°C for 10 hours to obtain rare earth fluoride-yttrium fluoride (YF 3 ).
[0023] figure 1 For the YF prepared by the method of this example 3 The X-ray diffraction spectrum is shown by figure analysis, and the prepared YF 3 Does not contain other impuritie...
Embodiment 2
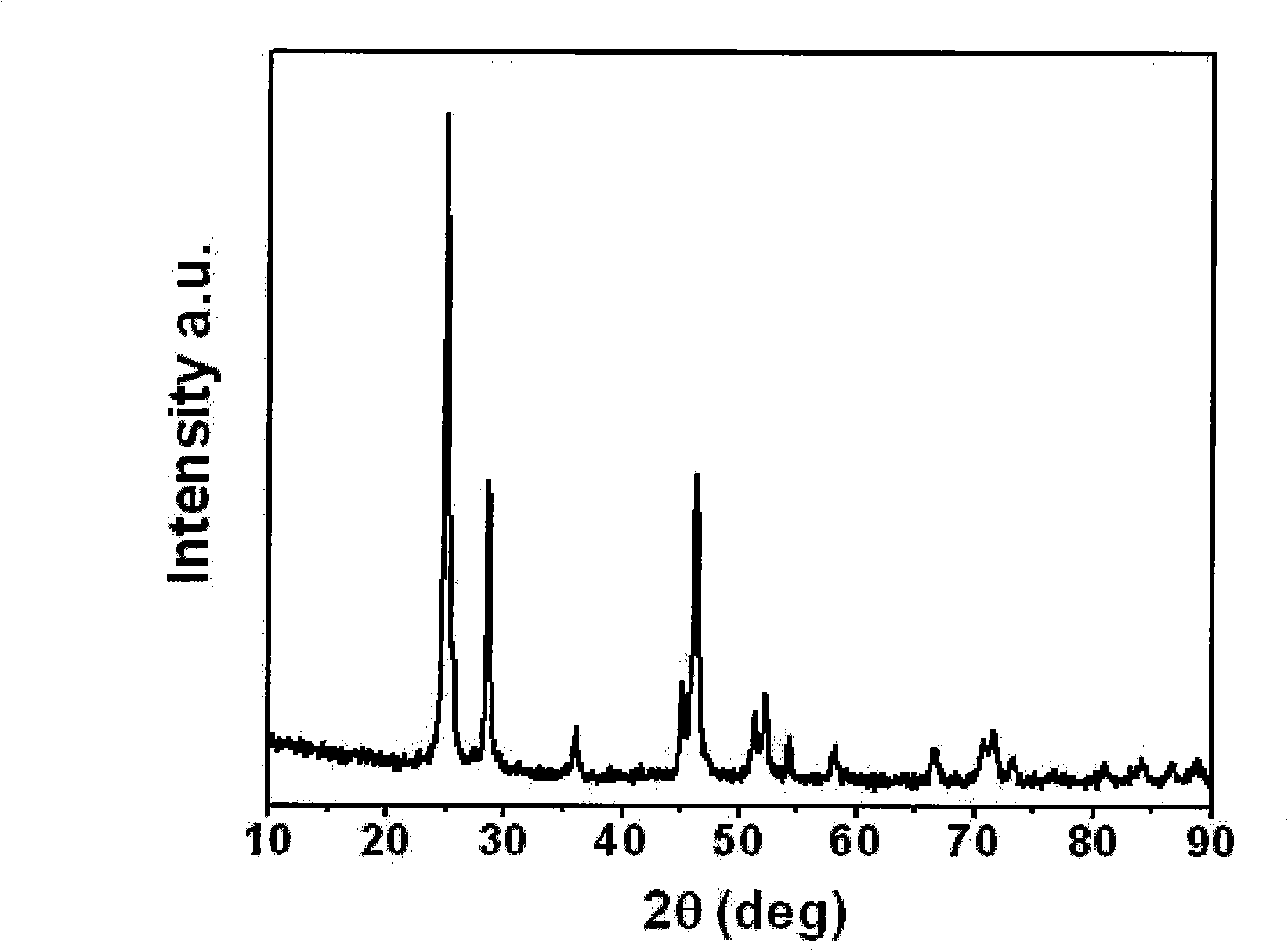
[0025] Lanthanum oxide (La 2 o 3 ) dissolved in 20% dilute acetic acid, La 2 o 3 The molar ratio with acetic acid is 1:7 to form a transparent aqueous solution of lanthanum acetate; ammonium fluoride (NH 4 F) dissolved in deionized water, NH 4 The dosage of F is La 2 o 3 6.5 times the molar amount, forming NH 4 F transparent aqueous solution; the aqueous solution of lanthanum acetate and NH 4 The F aqueous solution was mixed and reacted to form a slurry, which was ultrasonically mixed for 1 hour with an ultrasonic cleaner, and then lanthanum fluoride (LaF 3 ); the precipitated LaF 3 Put it in a vacuum drying oven, dry it at 100°C, and then sinter it in the air at 250°C for 8 hours to get LaF 3 .
Embodiment 3
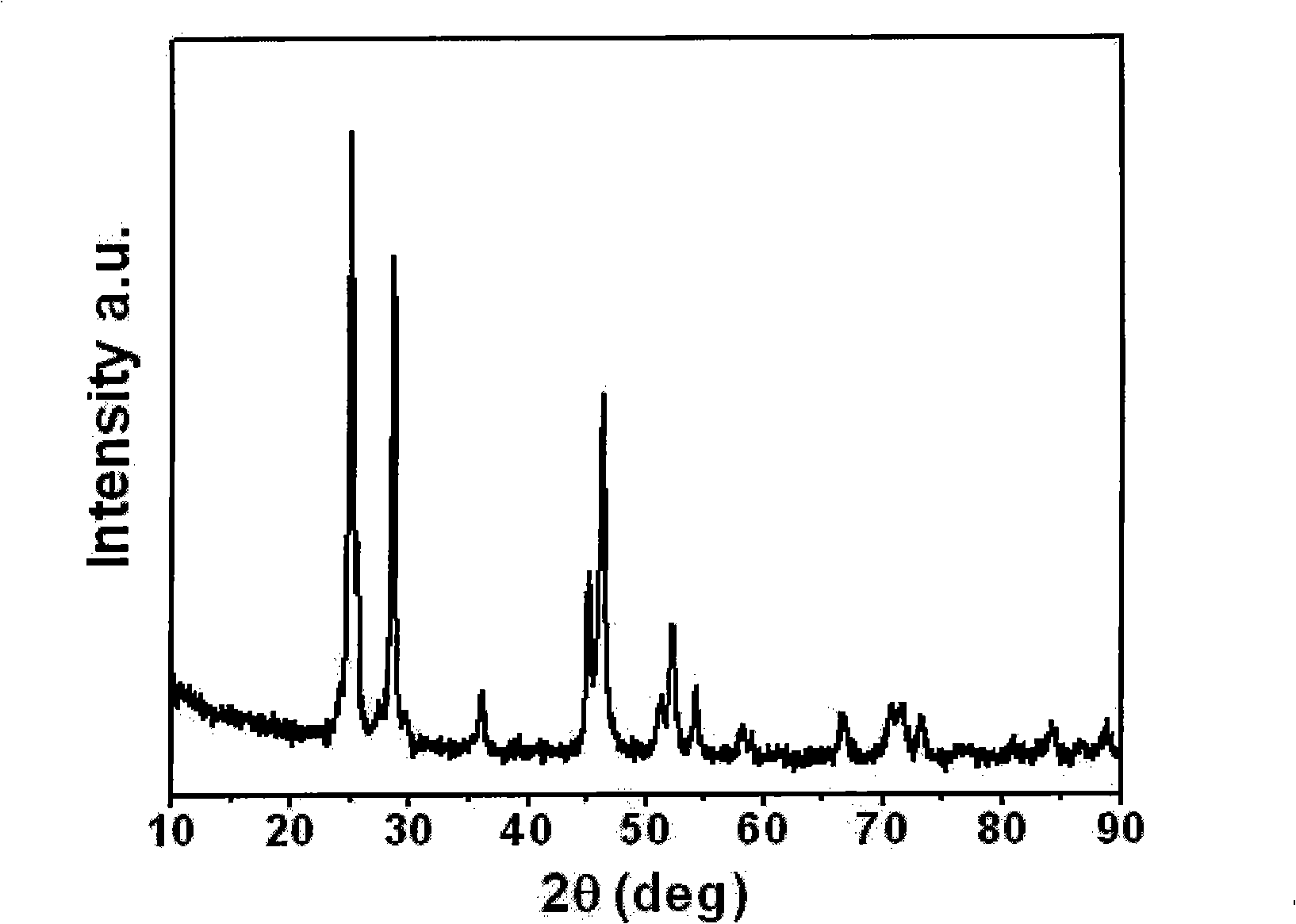
[0027] Praseodymium oxide (Pr 2 o 3 ) dissolved in 40% dilute acetic acid, Pr 2 o 3 The molar ratio with acetic acid is 1:8 to form transparent praseodymium acetate aqueous solution; ammonium fluoride (NH 4 F) dissolved in deionized water, NH 4 The dosage of F is Pr 2 o 3 6.2 times the molar amount, forming NH 4 F transparent aqueous solution; the aqueous solution of praseodymium acetate and NH 4 The F aqueous solution was mixed and reacted to form a slurry liquid, which was ultrasonically mixed for 0.75 hours with an ultrasonic cleaner, and then praseodymium fluoride (PrF 3 ); the precipitated PrF 3 Put it in a vacuum drying oven, dry it at 80°C, and then sinter at 350°C for 10 hours to get PrF 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com