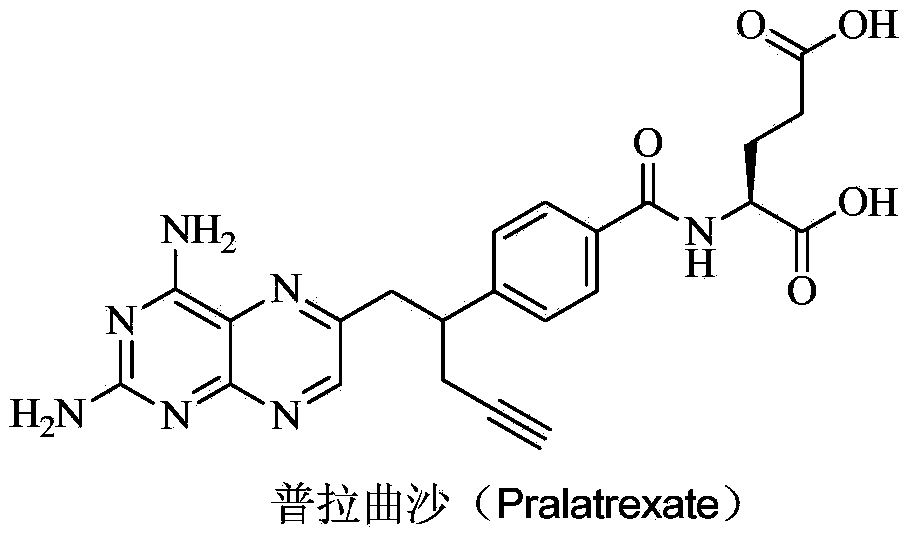
Pralatrexate degradation impurity and preparation method thereof
An impurity, pteridine-based technology, applied in the field of pralatrexate degrading impurities, can solve the problems of limited reaction degree, structural stability, difficult to degrade, separate, no compound synthesis report, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
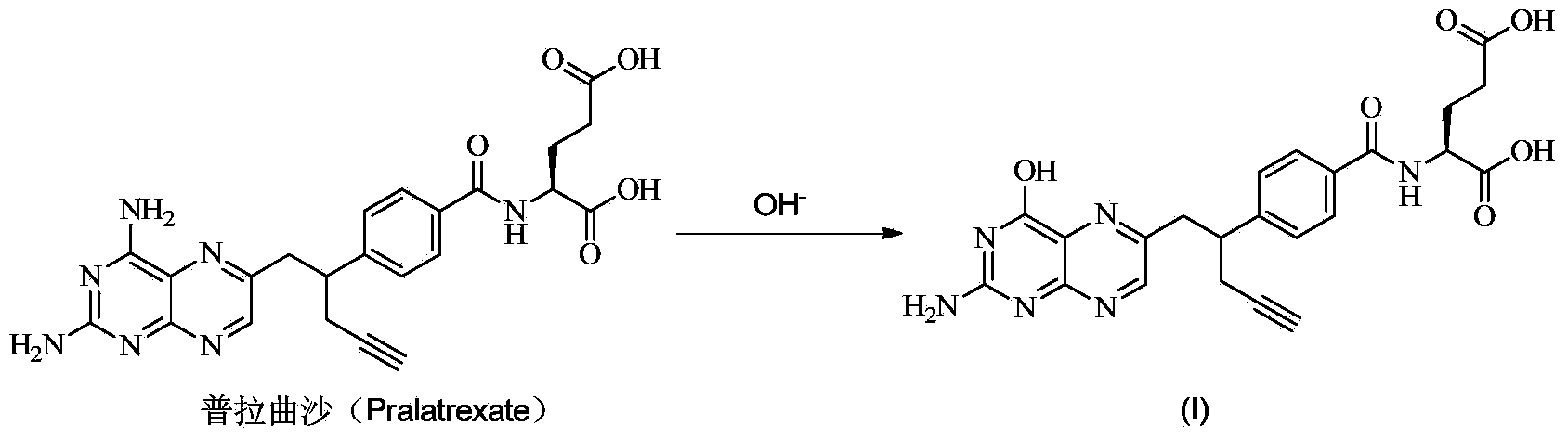
[0027] Add compound (II) (3.5g), sodium hydroxide (1.2g), water (30ml), and DMSO (30ml) into a 250ml reaction bottle, heat up to 60-65°C for reaction, HPLC detects that the reaction is complete, add isopropyl Alcohol (150ml), adjust the pH to 5-6 with glacial acetic acid, the solid precipitates, stir and beat for 1 hour, filter, wash the filter cake with water, and dry in vacuum for 16 hours to obtain 2.6g of compound (III) as a yellow solid, yield 74.1 %. TLC plate detection is single point, TLC conditions (developing solvent is dichloromethane:methanol:acetic acid=10:1:1, Rf=0.2).
Embodiment 2
[0029] Compound (III) (2.6g) and DMSO (14ml) were added into a 50ml reaction flask, and the temperature was raised to 25°C. Add L-glutamate dimethyl hydrochloride (1.9g), HOBt (1.5g), EDC (2.1g), add triethylamine (4ml), TLC monitors the completion of the reaction, pour the reaction solution into water (200ml ), the solid precipitated, stirred and beaten for 1 hour, filtered, the filter cake was washed with water, dried in vacuum for 16 hours, and purified by column chromatography (dichloromethane:methanol=30:1) to obtain 2.2g of compound (IV), which was light red Solid, yield 58.4%. The purity was 97% as detected by HPLC.
Embodiment 3
[0031] Add compound (IV) (2.2g), methanol (7ml), water (7ml) into a 25ml reaction flask. Add sodium hydroxide (0.5g), monitor the completion of the reaction by TLC, filter, adjust the pH of the filtrate to 5-6 with glacial acetic acid, solid precipitates, stir and beat for 1 hour, filter, wash the filter cake with water, and dry in vacuum for 16 hours to obtain the target compound 1.8g, as a light red solid, yield 86.6%. Through HPLC detection, the product purity is 96%.
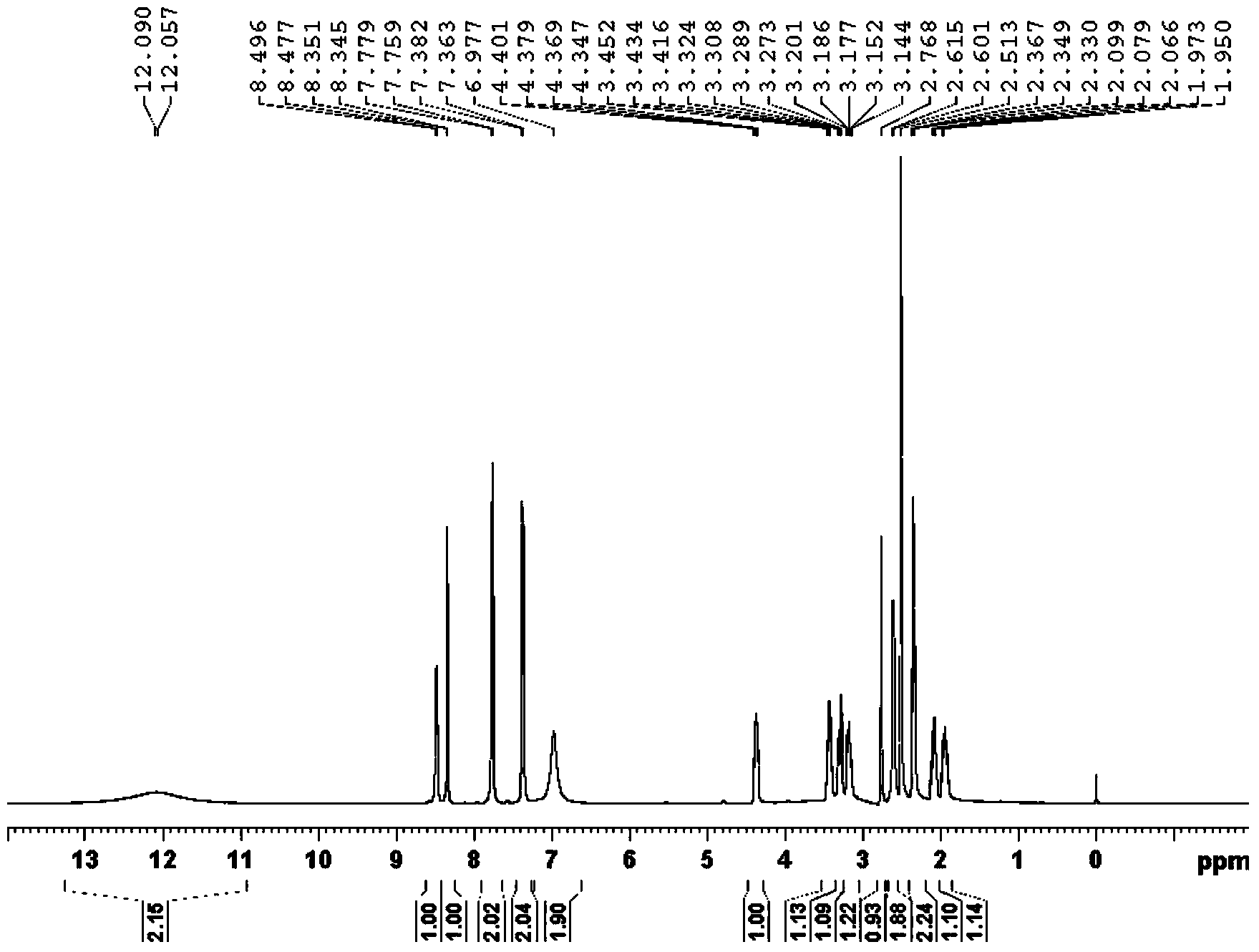
[0032] a. MS-ESI (m / z): 477.23[M-H]-;
[0033] b. 1 H-NMR (DMSO-d 6 )δ:12.07(br,2H),8.49(d,J=7.6Hz,1H),8.35(d,J=2.4Hz,1H),7.77(d,J=8.0Hz,2H),7.37(d, J=7.6Hz,2H),6.98(s,2H),4.40-4.35(m,1H),3.45-3.42(m,1H),3.32-3.27(m,1H),3.20-3.14(m,1H) ,2.77(s,1H),2.61(d,J=5.6Hz,2H),2.35(t,J=7.4Hz,2H),2.10-2.07(m,1H),1.97-1.92(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com