A kind of crystal form of pralatrexate and preparation method thereof
A technology of pralatrexate and its crystal form, which is applied in the field of medicine, can solve problems such as low crystallinity of the crystal form and difficulty in meeting the production requirements of the pharmaceutical industry, and achieve the effects of high crystallinity, simple preparation method, and mild crystallization conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 Preparation of pralatrexate D crystal form
[0037] Pralatrexate was prepared according to the preparation method described in the document "Synthesis and Antitumor Activity of 10-Propargyl-10-deazaaminopterin" J.MedChem.1993, 36:2228-2231 by DeGraw et al., with a purity of 97.01%.
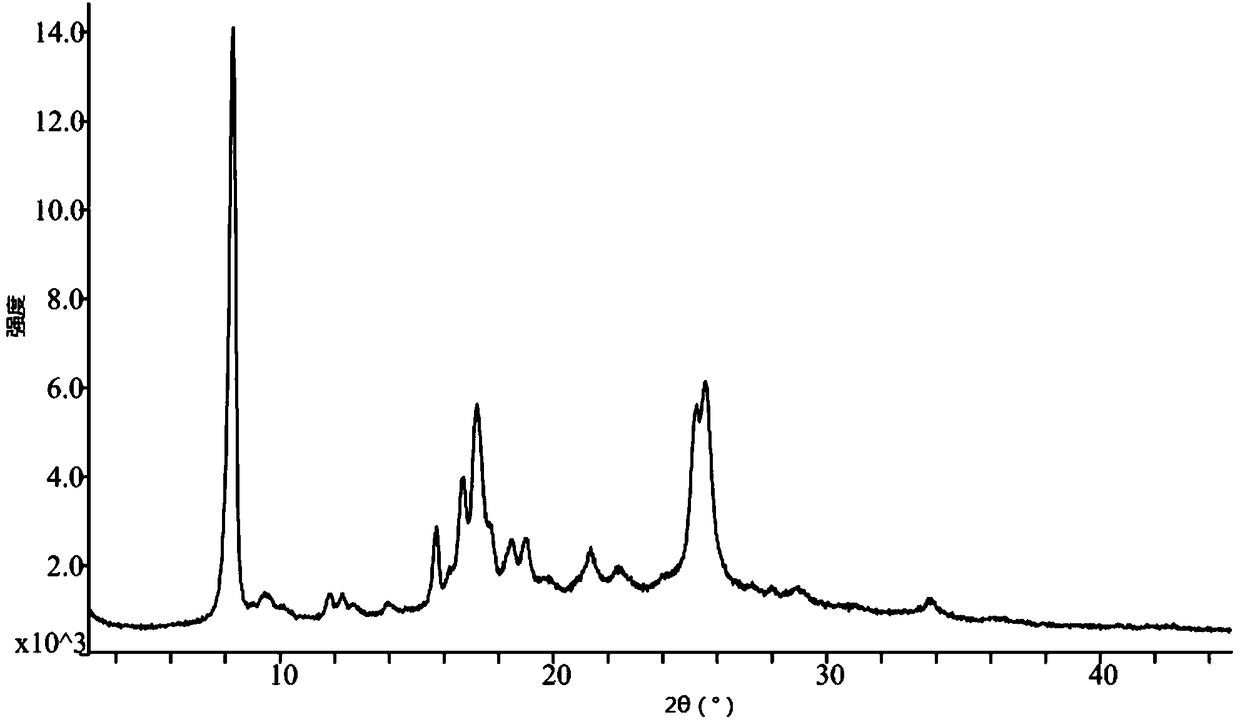
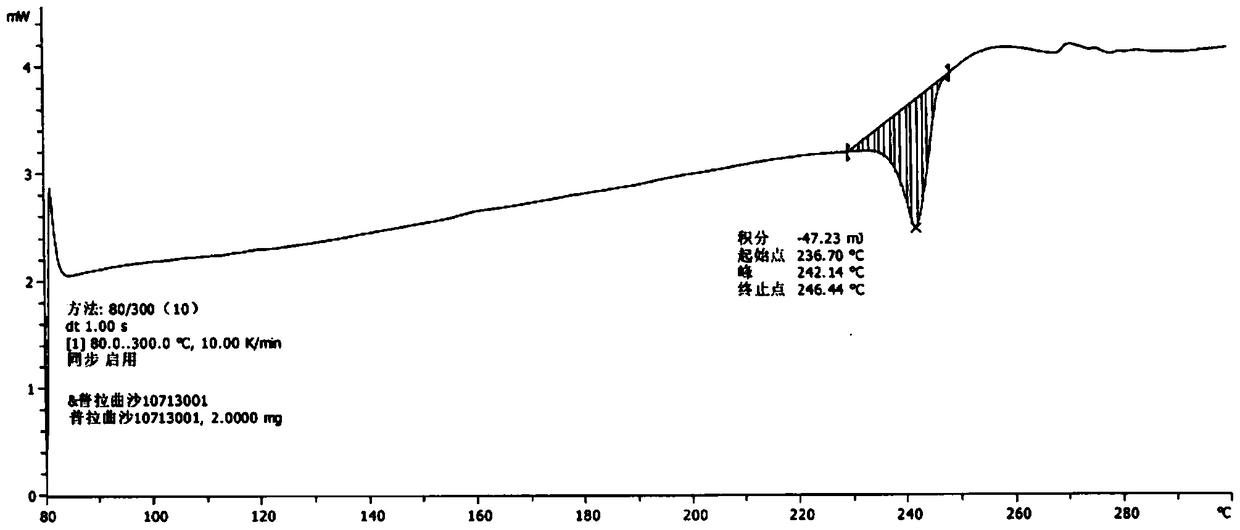
[0038] Take 15.0g of pralatrexate, add 100mL of N,N-dimethylformamide, raise the temperature to 50°C, stir for 10 minutes, add 50mL of acetonitrile dropwise, cool and crystallize naturally, keep warm at room temperature for 4 hours to continue the crystallization, After filtering, the filter cake was rinsed with 30 mL of acetonitrile, and the filter cake was vacuum-dried at 50° C. to obtain 12.1 g of pralatrexate Form D with a purity of 98.6%. it has figure 1 X-ray powder diffraction pattern shown.
Embodiment 2
[0039] Embodiment 2 Preparation of pralatrexate E crystal form
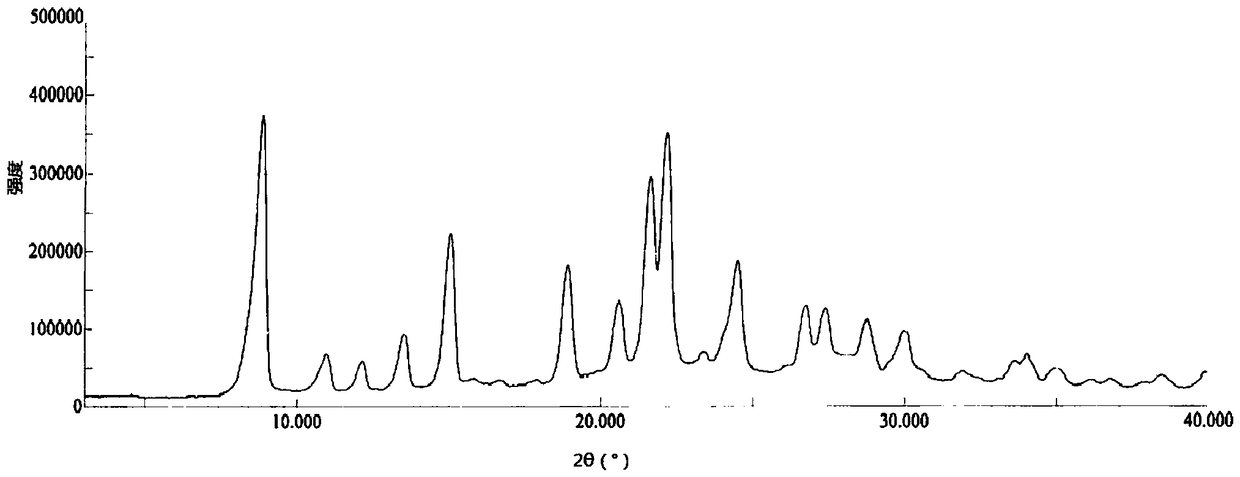
[0040] 1000mL reaction bottle, add 500mL ethanol aqueous solution (V 乙醇 :V 水 =3:2), heat up to 50°C, add 10.0g of pralatrexate crystal form D prepared in Example 1, stir until the solid dissolves, cool and crystallize naturally, keep warm at 25°C for 6 hours to continue the crystallization , filtered, the filter cake was rinsed with 20 mL of ethanol, and the filter cake was vacuum-dried at 60° C. for 30 hours to obtain 7.8 g of pralatrexate Form E.
Embodiment 3
[0041] Embodiment 3 Preparation of pralatrexate E crystal form
[0042] 500mL reaction bottle, add 250mL ethanol aqueous solution (V 乙醇 :V 水 =3:2), heat up to 80°C, add 10.0g of pralatrexate crystal form D prepared in Example 1, stir until the solid dissolves, cool down and crystallize naturally, and keep warm at 25°C for 6 hours to continue the crystallization , filtered, the filter cake was rinsed with 20 mL of ethanol, and the filter cake was vacuum-dried at 60° C. for 30 hours to obtain 8.6 g of pralatrexate E crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com