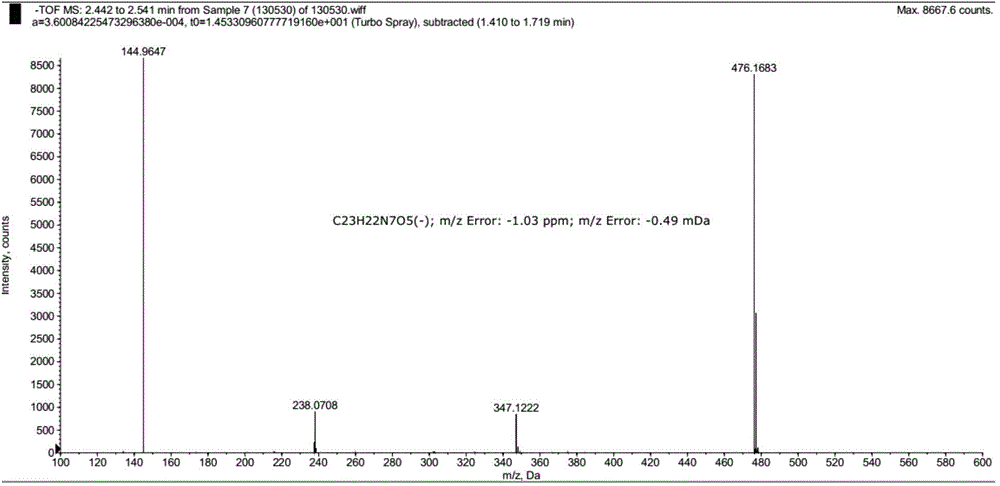
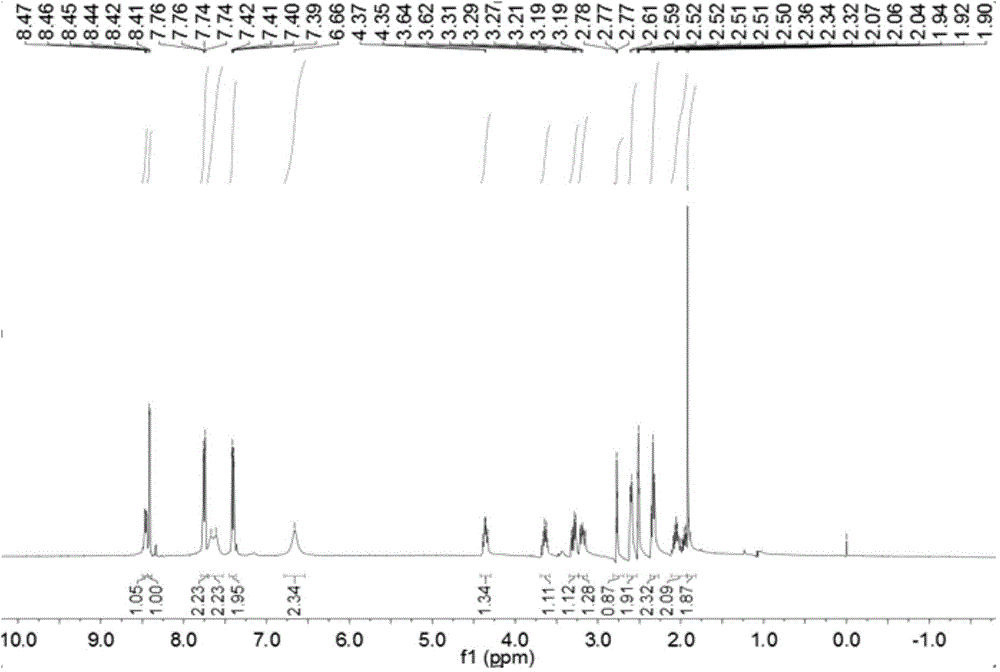
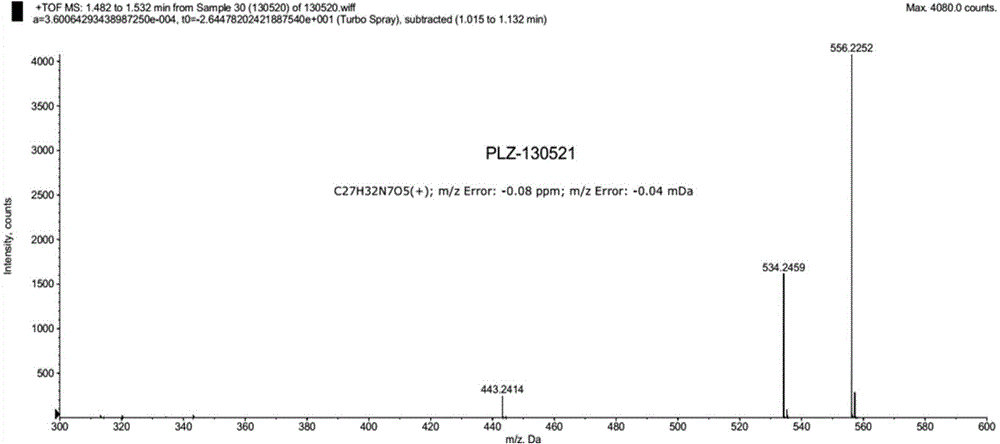
Pralatrexate preparation method
A technology of propargyl and diaminopteridine, applied in the direction of organic chemistry, etc., can solve the problems of environment, operator threat, unfavorable industrial production, high vacuum equipment, etc., to improve productivity and appearance, good industrial application prospect, Process green effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, the synthesis of 4-(2-carboxy-1-(2,4-diaminopteridin-6-yl)pent-4-yn-2-yl)benzoic acid
[0028]Embodiment 1. Take 1.02g of Compound I in a 100mL single-necked bottle, add 10mL of absolute ethanol, and after magnetically stirring for 5 minutes, add 10mL of purified water. After stirring for 5 minutes, add 10mL of 10% NaOH solution drop by drop. Stir, TLC monitors the reaction process, and the reaction ends after about 20 hours; add glacial acetic acid dropwise to adjust the pH to 6, remove ethanol by rotary evaporation, add 30 mL of purified water, adjust the pH to 6 with glacial acetic acid, and vacuum-dry the obtained solid at room temperature to obtain 0.59 g of the product. Yield 63%.
[0029] Embodiment 2. Take 10.30g of Compound I in a 1L single-necked bottle, add 100mL of methanol, stir magnetically for 5min, add 100mL of purified water, stir for 5min, add 100mL of 10% NaOH solution dropwise, and stir at room temperature after the addition is complete...
Embodiment 2
[0031] Example 2, Synthesis of 4-(1-(2,4-diaminopteridin-6-yl)pent-4-yn-2-yl)benzoic acid;
Embodiment approach 1
[0032] Embodiment 1. Heat the oil bath to 120oC in advance, take 6.2g of compound II in a 250mL single-mouth bottle, add 100mL DMSO, stir magnetically, put nitrogen in the oil bath after 5min, react under nitrogen atmosphere, and monitor the reaction process by TLC. The reaction ended after about 20 min. After the reaction solution was cooled to room temperature, it was poured into 500mL of purified water, and a large amount of yellow precipitates were precipitated immediately. After standing for 10 minutes, filtered, the filter cake was dissolved with dilute ammonia water, and the pH was adjusted to 6 with glacial acetic acid. The obtained precipitate was filtered, and the filter cake was dissolved with dilute ammonia water. Adjust the pH to 6 with glacial acetic acid, filter the resulting precipitate, and dry the filter cake under vacuum at room temperature to obtain 2.8 g of the product with a yield of 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com