A kind of high-purity pralatrexate solid and preparation method thereof
A solid-to-volume ratio technology, applied in the field of medicine, can solve problems such as difficulty in meeting the production requirements of the pharmaceutical industry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of pralatrexate D crystal form
[0052] Pralatrexate was prepared according to the preparation method described in the document "Synthesis and Antitumor Activity of 10-Propargyl-10-deazaaminopterin" J.Med.Chem.1993,36:2228-2231 by DeGraw et al., with a purity of 97.01% .
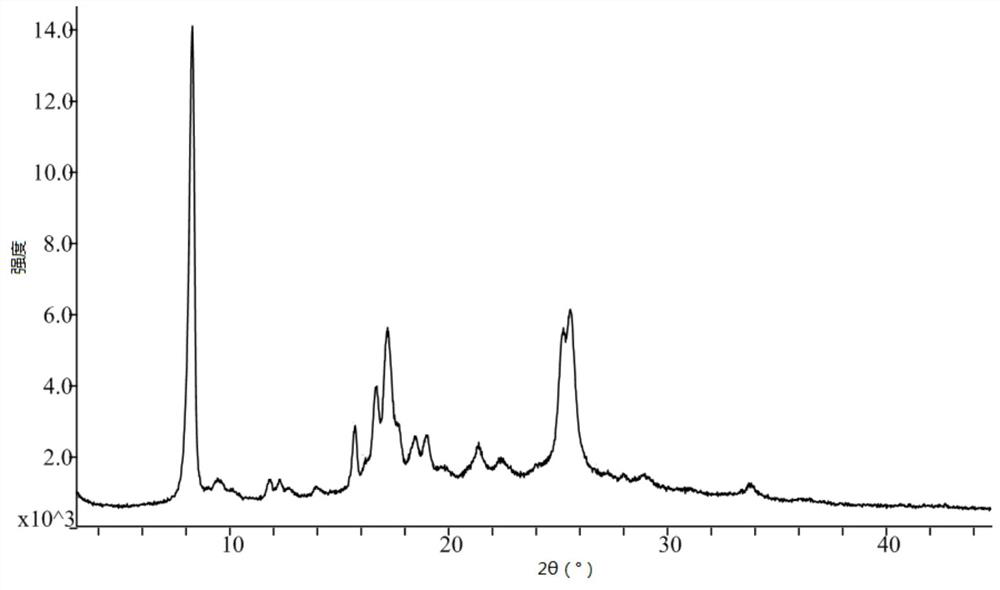
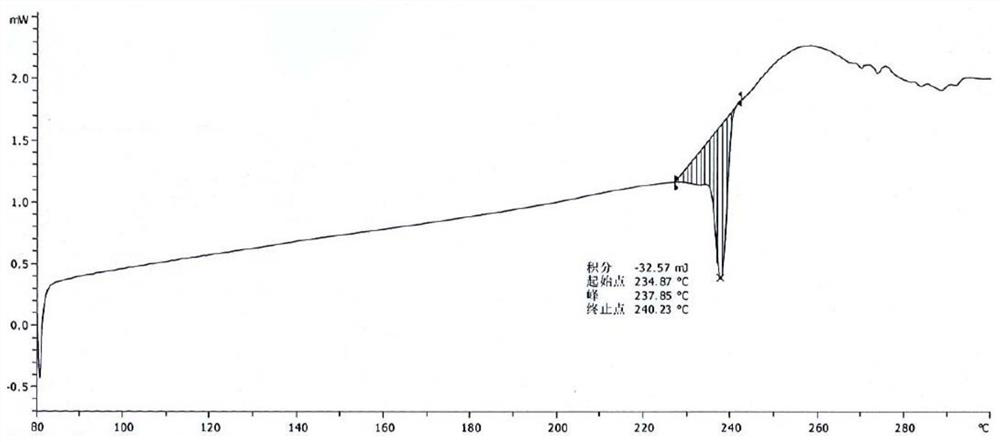
[0053] Take 15.0g of pralatrexate, add 100mL of N,N-dimethylformamide, raise the temperature to 50°C, stir for 10 minutes, add 50mL of acetonitrile dropwise, cool and crystallize naturally, keep warm at room temperature for 4 hours to continue the crystallization, After filtering, the filter cake was rinsed with 30 mL of acetonitrile, and the filter cake was vacuum-dried at 50° C. to obtain 12.1 g of pralatrexate Form D with a purity of 98.6%. it has figure 1 X-ray powder diffraction pattern shown. DSC: 197.3°C.
Embodiment 2
[0054] Example 2 Preparation of high-purity pralatrexate crystals
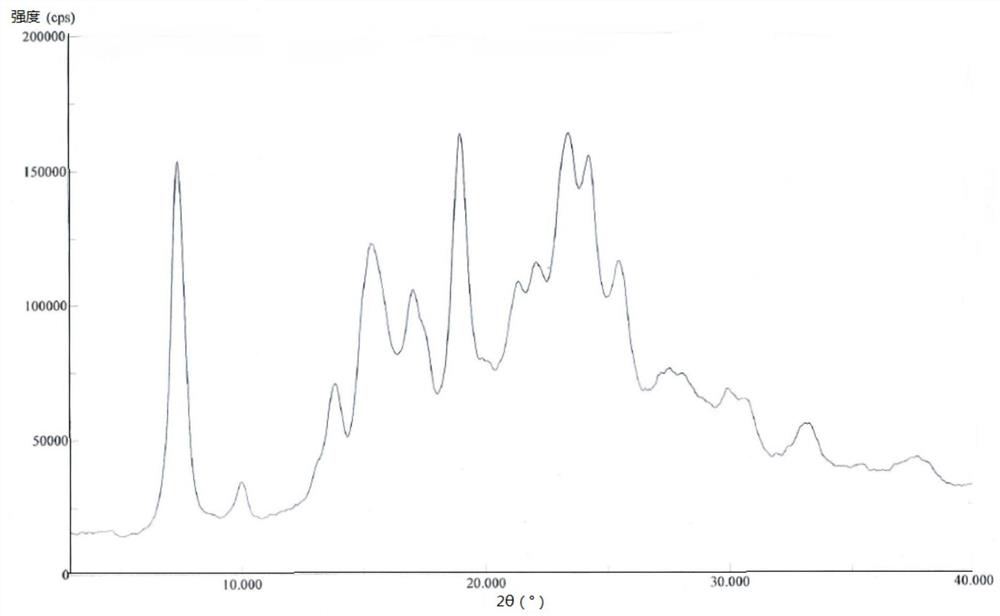
[0055] 1000mL reaction flask, add 500mL acetone aqueous solution (V 丙酮 :V 水 =3:2), heat up to 55-60°C, add 10.0g of pralatrexate D crystal form prepared in Example 1, stir until the solid dissolves, cool down naturally for 2 hours, and then cool down to 15±5°C Crystallize for 8 hours, filter, rinse the filter cake with 20 mL of ethanol, and vacuum-dry the filter cake at 60° C. for 30 hours to obtain 7.6 g of pralatrexate as a solid. DSC: 237.9°C.
Embodiment 3
[0056] Example 3 Preparation of high-purity pralatrexate crystals
[0057] 1000mL reaction flask, add 500mL acetone aqueous solution (V 丙酮 :V 水 =3:1), heat up to 65-70°C, add 10.0g of pralatrexate D crystal form prepared in Example 1, stir until the solid dissolves, cool down naturally for 2 hours, and then cool down to 15±5°C Crystallize for 8 hours, filter, rinse the filter cake with 20 mL of acetonitrile, and vacuum-dry the filter cake at 60° C. for 30 hours to obtain 7.8 g of pralatrexate as a solid. DSC: 238.8°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com