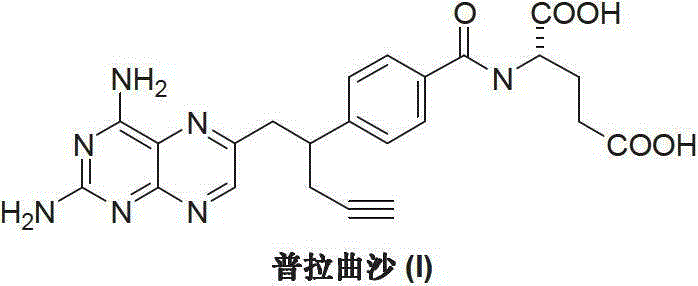
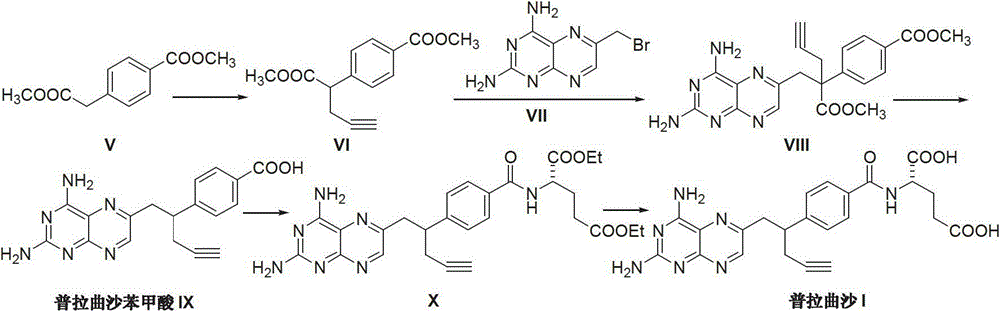
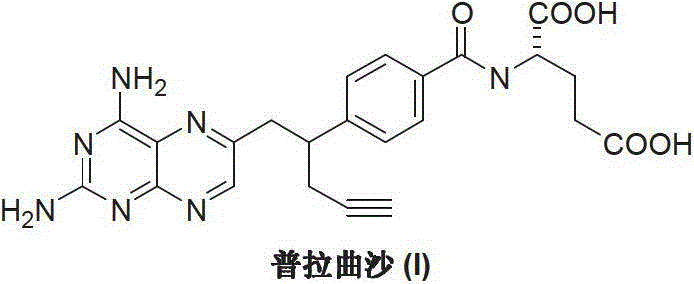
Preparation method of Pralatrexate
A preparation step and propargyl technology are applied in the field of organic synthesis route design and the preparation of raw materials and intermediates, which can solve the problems of difficult separation, rare raw materials, and high cost, and achieve the advantages of simple process, easy availability of raw materials, and promotion of development. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add N-[4-(1-(2-propynyl)-3,4-dioxo-n-butyl)benzoyl]-L-glutamic acid dimethyl ester (II) (4.01 g, 10 mmol), 30 mL of 1M sodium hydroxide solution and 30 mL of distilled water, stirred at room temperature for 3 hours, added activated carbon for decolorization for 30 minutes, and filtered. The filtrate was adjusted with 2N hydrochloric acid to adjust the pH value of the reaction system to 2-3, and the reaction was stirred for 2 hours. After extraction with dichloromethane, the extract was dried and evaporated to dryness to obtain a light yellow oily substance N-[4-(1-(2-propynyl)-3,4-dioxo-n-butyl)benzoyl] - 3.3 g of L-glutamic acid (IV), the yield is 88.5%.
Embodiment 2
[0023] Add N-[4-(1-(2-propynyl)-3,4-dioxo-n-butyl)benzoyl]-L-glutamic acid (IV) (2.24g, 6mmol ), acetone oxime (5.01g, 7mmol) and 50mL of distilled water, 1N dilute hydrochloric acid was added dropwise to adjust the pH value of the reaction system to 2-3. The temperature was raised to 50-60°C, and the reaction was stirred for 2 hours. Cool down to room temperature, add 2,4,5,6-tetraaminopyrimidine (III) (0.70 g, 5 mmol), react at room temperature for 4 hours, then raise the temperature to reflux for 6 hours, and a precipitate appears. Cooling, crystallization, and filtration gave 1.92 g of off-white solid pralatrexate (I), with a yield of 80.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com