Crystal or amorphous substance of steroid derivative fxr agonist, preparation method and use thereof
一种结晶、溶剂的技术,应用在药物化学领域,能够解决无法激活小鼠和人类FXR、内源性FXR配基自然性不确定等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] The preparation of embodiment 1 formula I compound
[0095] The preparation of step 1-1 formula 3 compound
[0096]
[0097] Methanol (33 L) was added to a 50 L reaction kettle at 25 degrees Celsius, substrate 2 (3.330 kg, 8.23 mol) was added to the reaction kettle, and p-toluenesulfonic acid monohydrate (156.6 g, 0.823 mol) was added , the reaction was heated to 60 °C and stirred at this temperature for 12 hours. TLC monitored the reaction, showing disappearance of starting material. HPLC showed about 100% product formation. The reaction solution was cooled to room temperature, then the pH value was adjusted to about 9 with saturated sodium bicarbonate solution, and the solution was spin-dried to obtain the crude product. The crude product was dissolved in ethyl acetate (30 L), followed by saturated sodium bicarbonate solution (9 L), water (9L), washed with saturated brine (9L). The organic phase was spin-dried to obtain the product. The product is brown oily...
experiment example 1
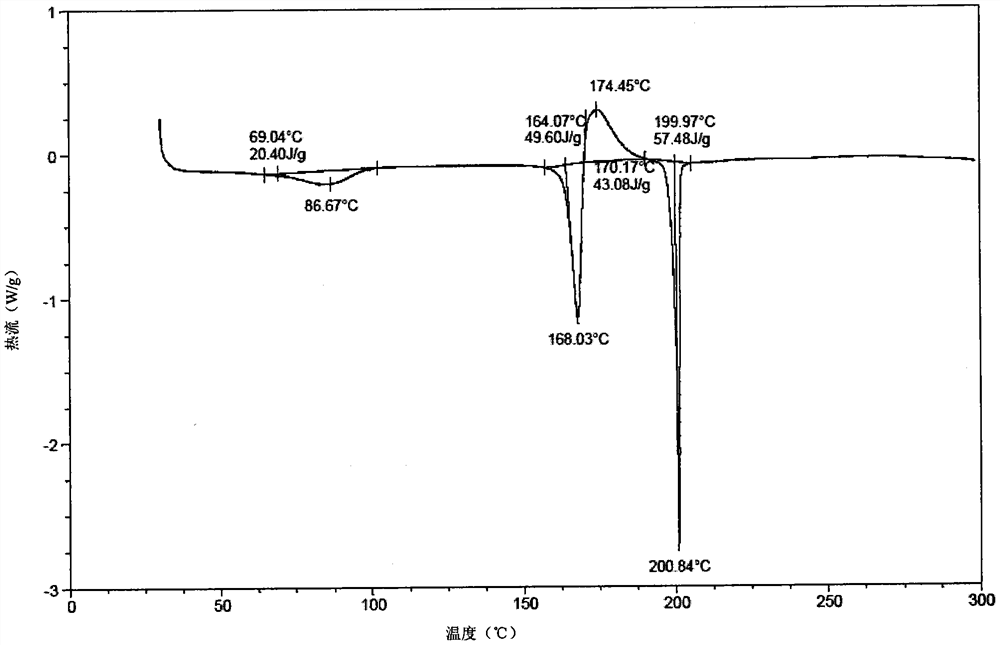
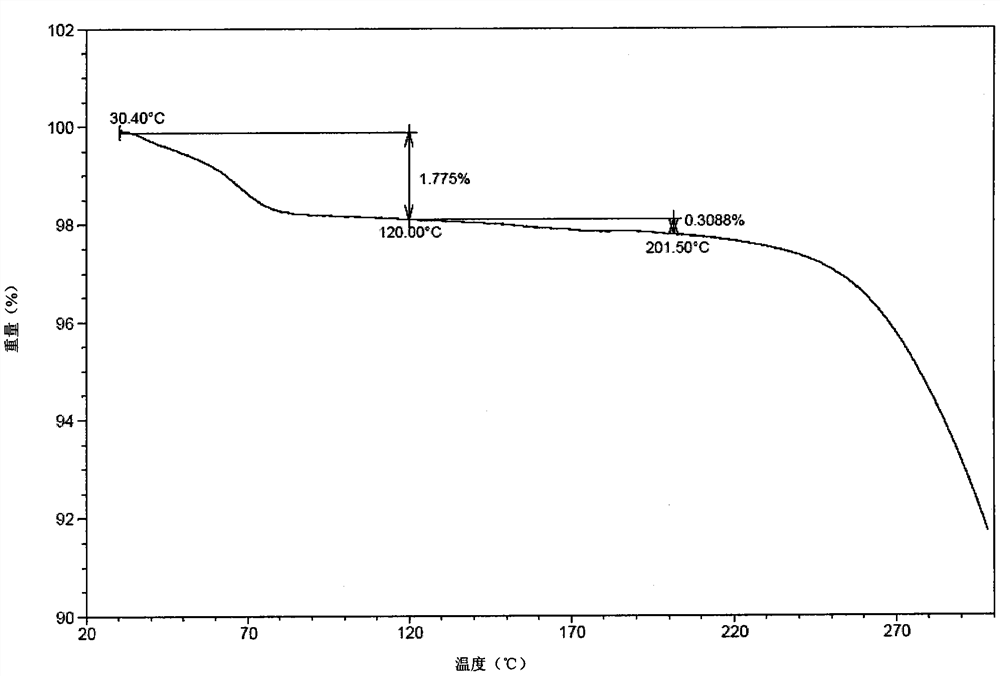
[0135] The solid stability test of experimental example 1 crystal A
[0136] Investigate crystal A under the following conditions: 1) 40°C (open), 2) 60°C (open), 3) room temperature / 92.5%RH (open), 4) room temperature / 75%RH (open), 5 ) 40°C / 75%RH (open), 6) solid stability at 60°C / 75%RH (open). The room temperature is selected from 20°C to 30°C.
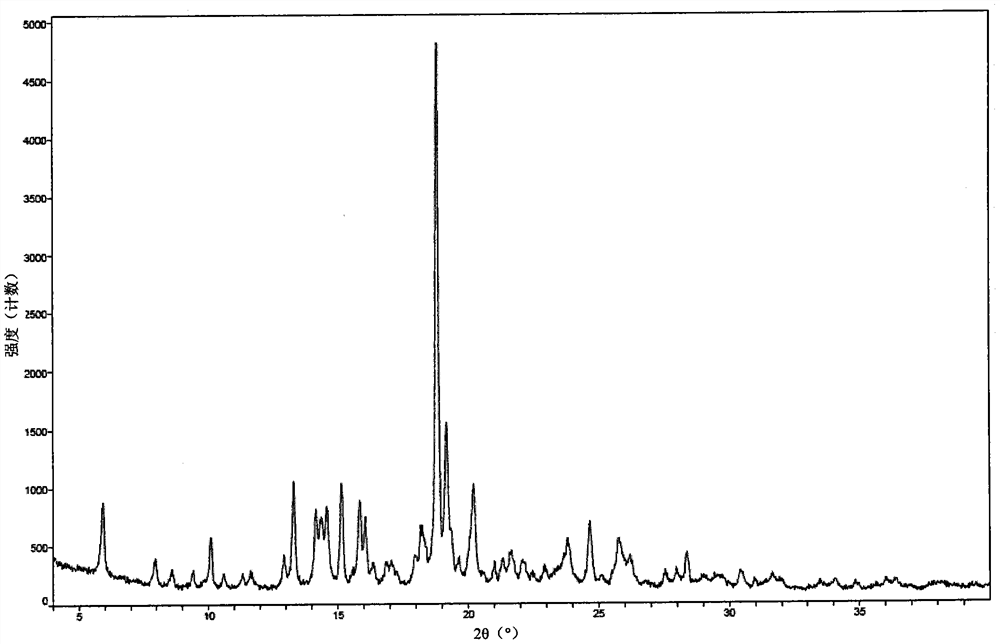
[0137] Weigh an appropriate amount of crystal A sample and place it on the bottom of the glass sample bottle, and spread it into a thin layer. The samples placed under all the above conditions were sealed with aluminum foil, and some small holes were punched on the aluminum foil to ensure that the samples could fully contact with the ambient air. Samples were taken on the 5th and 10th day for XRPD detection, and the test results were consistent with the initial The detection results were compared, and it was found that the crystal forms of the samples did not change.
experiment example 2
[0138] The solid stability test of experimental example 2 crystal B
[0139] Investigate crystal B under the following conditions: 1) room temperature / 92.5% RH (open), 2) room temperature / 75% RH (open), 3) 40°C / 75%RH (open), 4) 60°C / 75 Solid stability in % RH (open). The room temperature is selected from 20°C to 30°C.
[0140] Weigh an appropriate amount of crystal B sample and place it on the bottom of the glass sample bottle, and spread it into a thin layer. The samples placed under all the above conditions were sealed with aluminum foil, and some small holes were punched on the aluminum foil to ensure that the samples could fully contact with the ambient air. Samples were taken on the 5th and 10th day for XRPD detection, and the test results were consistent with the initial The detection results were compared, and it was found that the crystal forms of the samples did not change.
[0141] The solid stability test of the solid amorphous of the compound shown in Experiment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com