Polymorphic substance of oxycodone hydrochloride as well as preparation method and application of polymorphic substance
A technology for oxycodone hydrochloride and polymorphs, which is applied in the field of polymorphs of oxycodone hydrochloride and its preparation, can solve problems such as agglomeration, discoloration, and increased impurities, and achieve a simple preparation method , mild crystallization conditions, and excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
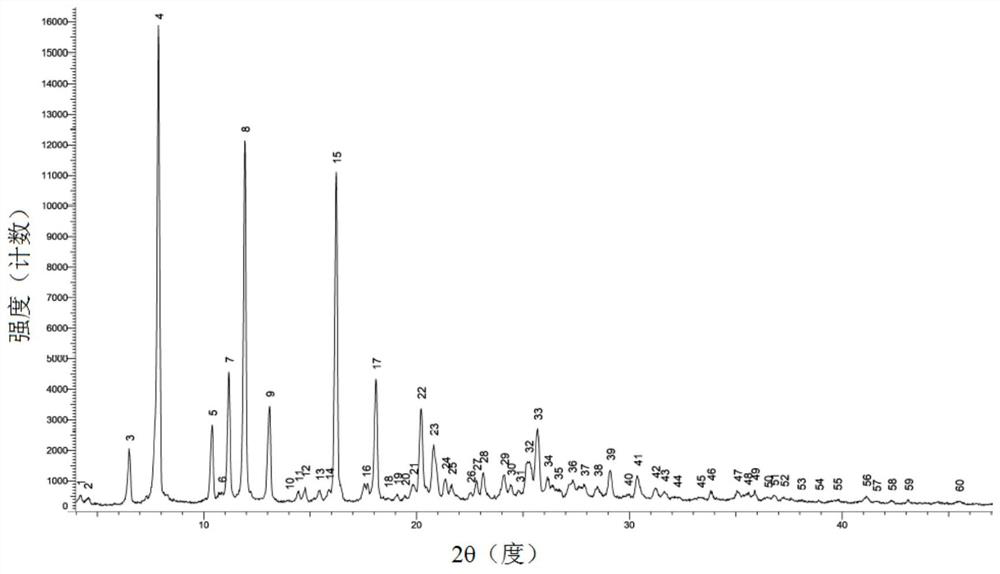
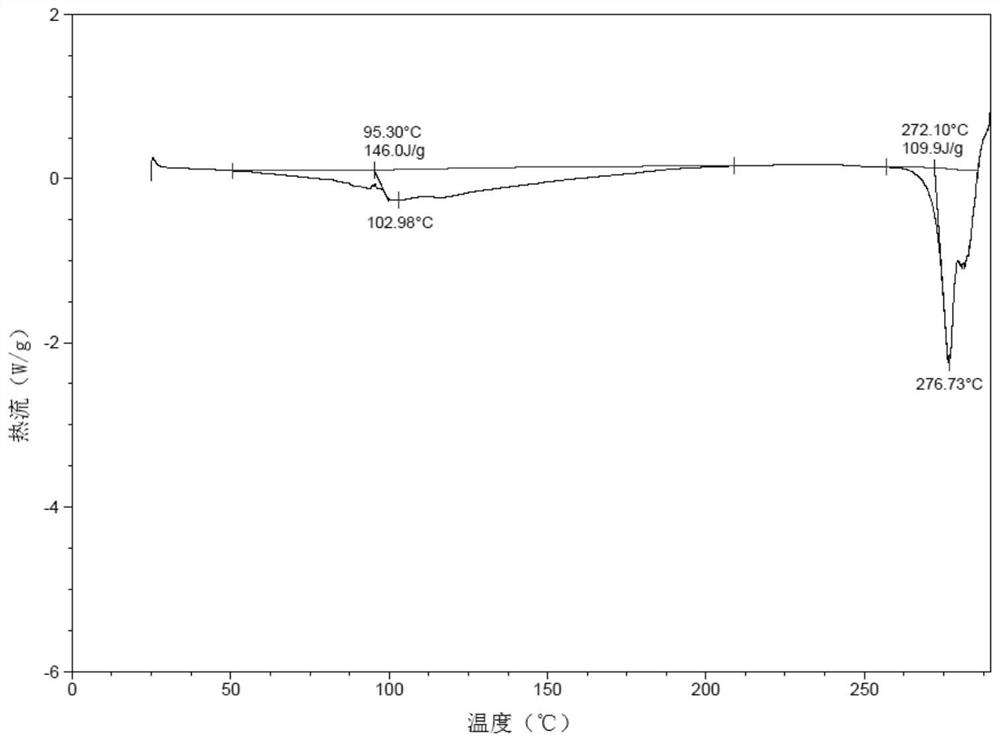
[0099] The XRPD spectrogram of the polymorphic form of oxycodone hydrochloride in embodiment 1, embodiment 2, embodiment 3 and embodiment 4 is respectively as figure 1 , Figure 8 , Figure 10 and Figure 12 As shown, the XRPD data are shown in Table 1, Table 2, Table 3 and Table 4 respectively. In order to test the stability of the polymorph, the polymorph of oxycodone hydrochloride with crystal form α in Example 1 was stored for 36 months at 25°C±2°C / 65%RH±5%. XRPD tests were conducted in 0, 6, 12, 24 and 36 months, the results are as follows Figure 6 and Figure 7 shown.
[0100] Table 1 XRPD data for polymorphs of oxycodone hydrochloride with Form α
[0101] serial number Signal peak number 2θ[°] D value (Angstrom) Relative Strength(%) 1 3 6.50 13.58 11.2 2 4 7.88 11.22 100.0 3 5 10.40 8.50 16.0 4 7 11.18 7.91 27.1 5 8 11.94 7.41 75.9 6 9 13.10 6.76 20.0 7 15 16.23 5.46 69.2 8 17 18.10 ...
Embodiment 2
[0172] Example 2: Preparation of Oxycodone Hydrochloride Polymorphs with Crystal Form β
[0173] Take 0.5 g of the polymorph obtained in Example 1 and spread it on a watch glass with a thickness of 1 mm, and place it in an environment with a temperature of 10-30° C. and a humidity of 70%-80% for 4 hours to obtain needle-shaped crystals.
[0174] Tested by XRPD, the results are as follows Figure 8 As shown, it was determined to be the crystal form β.
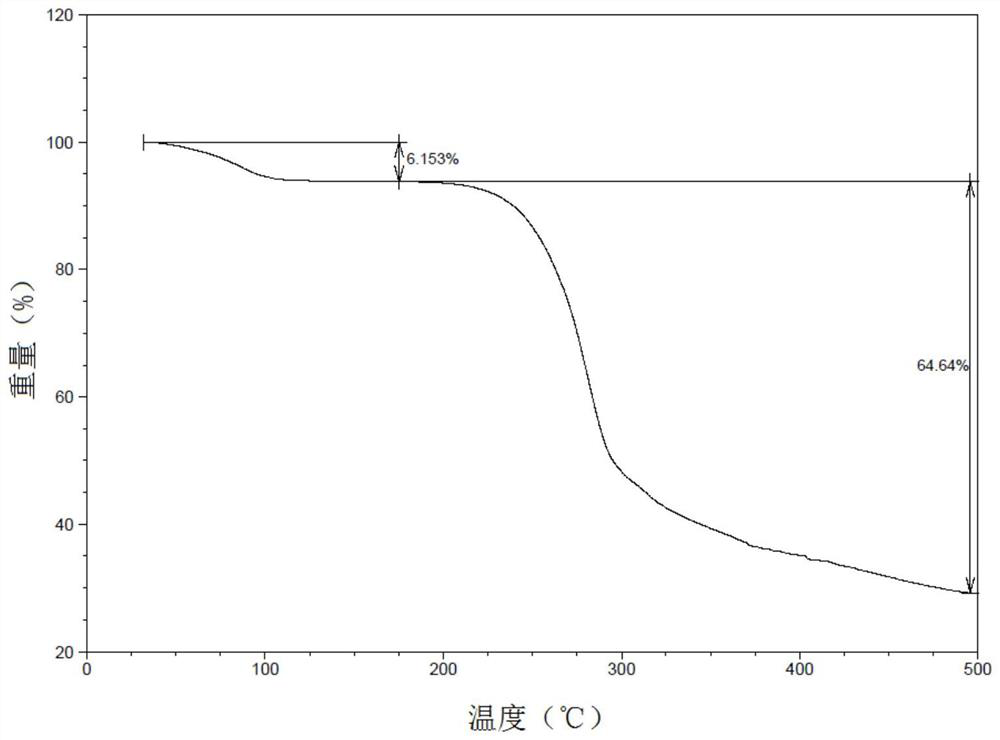
[0175] After thermogravimetric analysis, the results were as follows Figure 9 As shown, under the set test conditions, the weight loss of the sample presents two stages: the initial temperature of the first stage is about 80°C, and the weight loss of this stage ends at about 160°C, and the weight loss rate is 10.17%. This stage is caused by endothermic Water volatilizes and loses weight; the initial temperature of the second stage is about 160°C, and it loses weight rapidly as the temperature increases, and ends at about 320°...
Embodiment 3
[0176] Example 3: Preparation of Oxycodone Hydrochloride Polymorphs with Form γ
[0177] Take 0.5 g of the polymorph obtained in Example 1 and spread it on a watch glass with a thickness of 1 mm, place it in a decompression drying oven with a pressure of 0.08-0.1 MPa, and dry it for 4 hours at a temperature of 85-95 ° C to obtain Dark white powdery crystals.
[0178] Tested by XRPD, the results are as follows Figure 10 As shown, it was determined to be the crystal form γ.
[0179] After thermogravimetric analysis, the results were as follows Figure 11As shown, under the set test conditions, the weight loss of the sample presents three stages: the initial temperature of the first stage is about 200°C, and the weight loss is rapid as the temperature rises, and the weight loss rate is 43.21% at about 275°C; The second stage continues to lose weight rapidly, and the weight loss rate is 38.75% at about 345 °C; the third stage becomes gentle with the increase of temperature, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com