A kind of crystal form of bortezomib, its preparation method and its pharmaceutical composition and application
A technology of bortezomib and composition, applied in the field of medicinal chemistry, can solve the problems of unfavorable liquid preparation preparation, weak antioxidant capacity, poor solubility of bortezomib crystal form, etc., achieves good clarity and visible foreign matter, and fast dissolution rate , the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] The preparation of the crude bortezomib compound used in the present invention can refer to Chinese invention patent CN1960996.
[0069] Among them, amorphous bortezomib, bortezomib FormI and FormII can be purchased from the market, or can be prepared by existing methods, for example, refer to WO2008075376A1 to prepare FormI and FormII, and refer to US5780454 to prepare amorphous bortezomib.
Embodiment 1
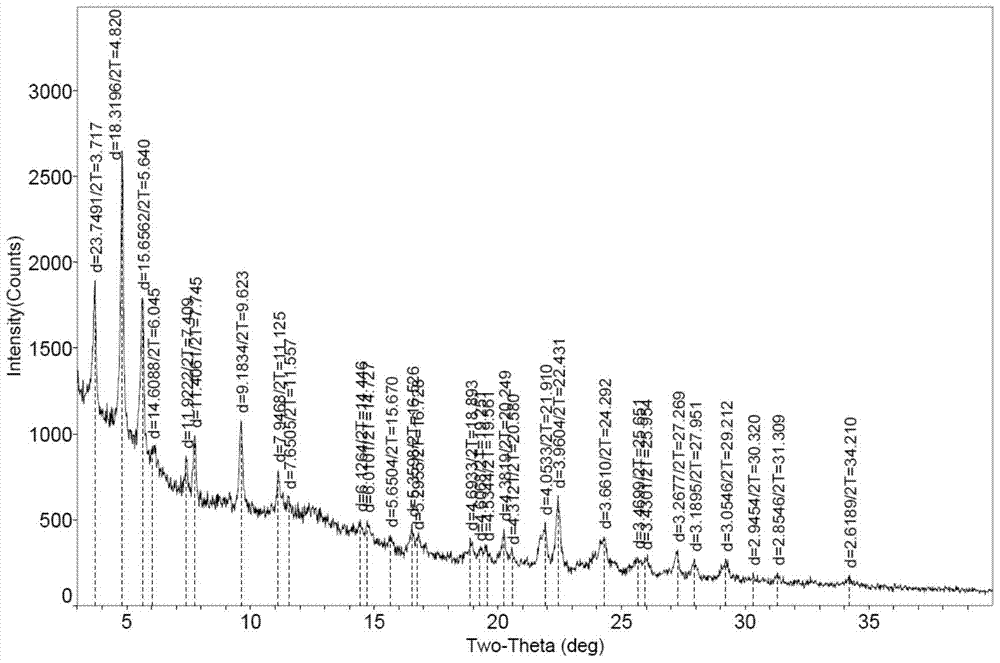
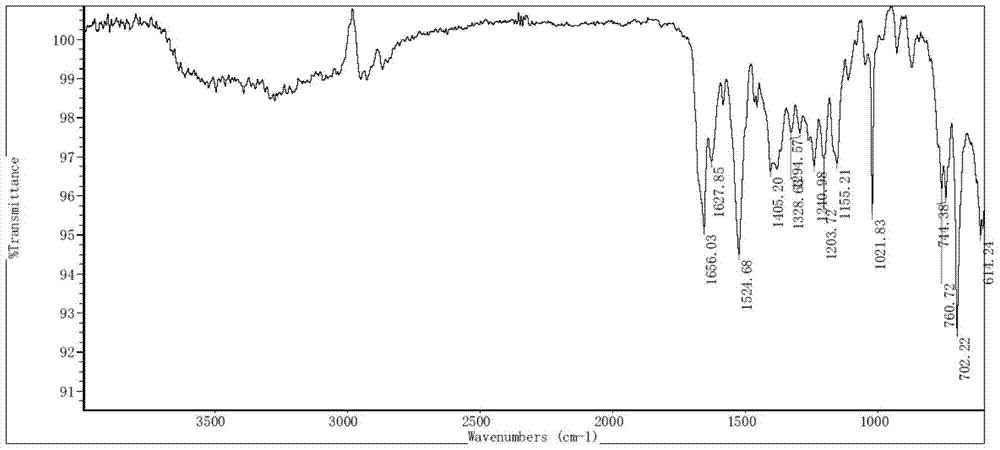
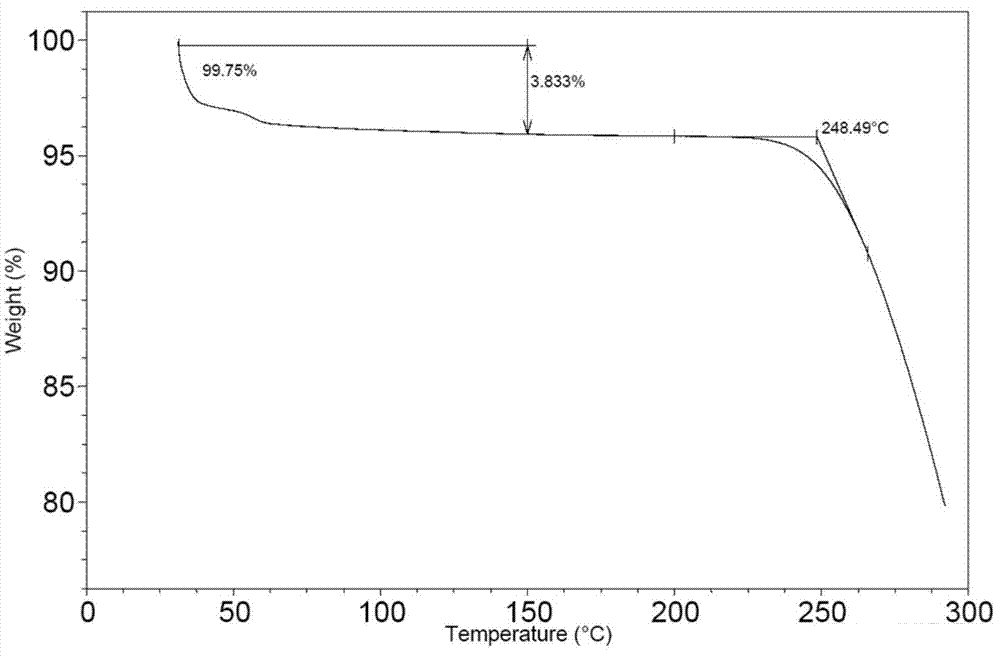
[0072] Put 100mg of bortezomib Form I in a 5ml glass vial, add 2ml of ethyl acetate, stir the crystal slurry at room temperature for 6 hours, centrifuge, and vacuum dry at 40°C for 4 hours to obtain bortezomib Form F, the purity of which was 99.6% by HPLC. The X-ray powder diffraction (XRD) pattern of this crystal form is shown in figure 1 ; IR spectrum see figure 2 ; TGA spectrum see image 3 , the weight loss of Form F is about 3.8% before 150°C, and the decomposition temperature is about 248°C; see the PLM spectrum Figure 4 ; DVS map see Figure 5 , see 6 for the before and after comparison of oxidation stability XRD.
Embodiment 2
[0074] Put 50mg of bortezomib Form I in a 5ml glass vial, add 2ml of ethyl acetate, stir the crystal slurry at room temperature for 4 hours, centrifuge, and vacuum dry at 40°C for 6 hours to obtain bortezomib Form F, the purity of which was 99.5% by HPLC. The X-ray powder diffraction (XRD) collection of patterns and figure 1 Basically the same, other spectrograms are consistent with those of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com