A kind of preparation method of pyrrole compound
A compound and mixture technology, applied in the field of preparation of pyrrole compounds, can solve problems such as difficult industrial production and inconvenient implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
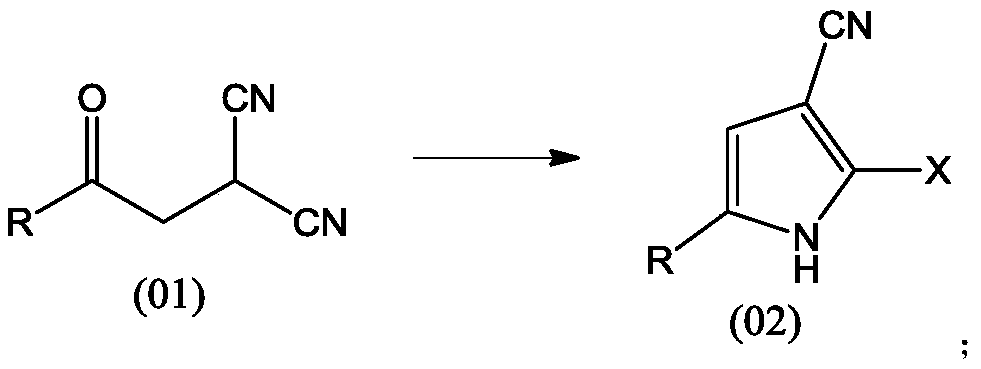
[0007] The invention provides a method for preparing pyrrole compounds, the reaction conditions of which are simple and easy to operate and control, and are suitable for industrial production. In the method provided by the present invention, a preparation method of pyrrole compound (02), comprising: reacting compound (01) with a halogenating reagent in an organic solvent to obtain compound (02),
[0008]
[0009] Among them, R is C1-C10 (1 carbon to 10 carbons) straight chain or branched chain alkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, such as methyl, ethyl, isopropyl base, n-butyl, isobutyl, cyclopropyl, pyridyl, phenyl, etc., R is optionally substituted by halogen, and the halogen is fluorine, chlorine, bromine or iodine; X is chlorine or bromine.
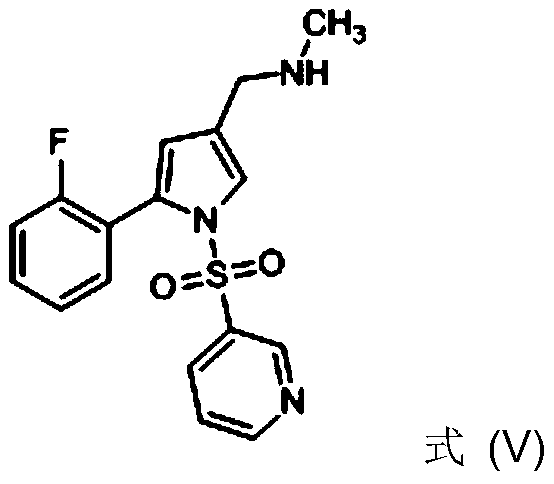
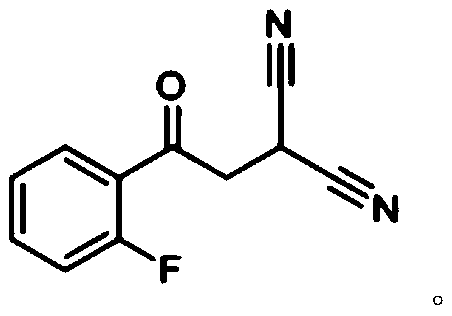
[0010] In some embodiments, R is phenyl. In some embodiments, R is ethyl. In some embodiments, R is 2-fluorophenyl.
[0011] In some embodiments, X is chlorine. In some embodiments, X is bromine.
[0012] The ...
specific Embodiment approach
[0038] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0039] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0040] In the present invention, g means gram, mL means milliliter, mol / L means mole / liter, and h means hour.
[0041] In the following examples, the compound shown in the formula (01) shown in the following reaction formula is compound (01), and the compound shown in formula (02) is compound (02):
[0042]
[0043] When R in the present invention is other groups, please refer to the methods in the following examples to prepare other compounds.
[0044] In the present invention, the completion of the reaction means that the remaining amount of the raw material compound (01) is not higher than 5%...
Embodiment 1
[0047] Add 6.17 g of compound (01) to the reaction flask at room temperature, then add 30 mL of homemade 4.07 mol / L ethyl acetate hydrochloride solution, control the temperature at 35°C-45°C, react for 2 hours, and the reaction is complete; the reaction solution is concentrated under reduced pressure to Dry, mix the residue with 24.68mL of ethyl acetate, heat to reflux, and keep at reflux for 1h; then lower the temperature to 45°C and keep warm for 0.5h, then lower the temperature to 30°C and keep warm for 0.5h, then cool down to 0°C and keep warm for 0.5h; After filtration, the obtained solid was dried under reduced pressure at 60° C. to obtain compound (02): 4.80 g of solid with a purity of 96.5% and a yield of 71%. After comparing and analyzing the test results before the post-treatment of the reaction solution and the test results of the product, it was found that an unknown impurity (impurity 1) with a higher content was generated during the reaction process, with a conten...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com