Guanidinylation SS-PAAs polymer as well as preparation and application thereof
A technology of polyaminoamine and guanidinylation, which is applied in the direction of gene therapy, genetic material composition, and the use of vectors to introduce foreign genetic material, etc., to achieve the effect of improving transfection efficiency and reducing cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
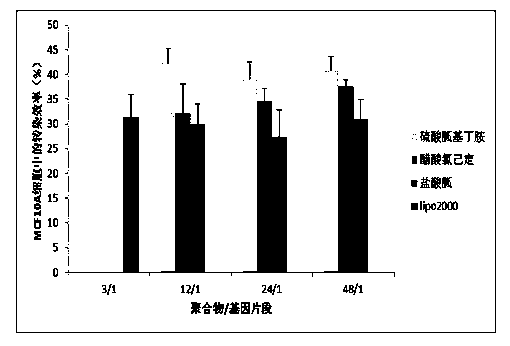
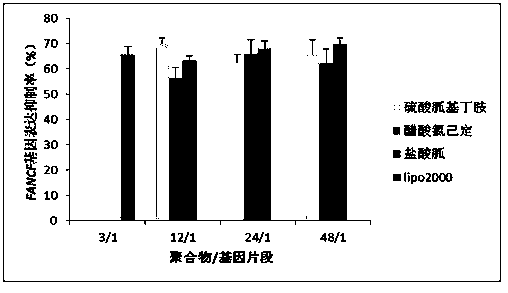
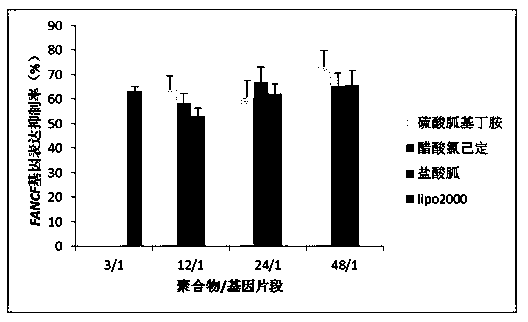
[0063] Example 1: Preparation of SS-PAAs with Agmatine Sulfate as Guanylation Reagent
[0064] Materials and Methods
[0065] 2,2,4,6,7-Pentamethyldihydrobenzofuran-5-sulfonyl chloride (Pbf-Cl): purchased from Jill Biochemical (Shanghai) Co., Ltd. N,N-bis(acryloyl)cystamine (CBA): purchased from Alfa Aesar (Tianjin) Chemical Co., Ltd. Agmatine sulfate: purchased from Changzhou Archie Biotechnology Co., Ltd. Trifluoroacetic acid (TFA): purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. pcDNA3.1-EGFP-cDNA plasmid and plasmid extraction kit: purchased from Tiangen Biochemical Technology Co., Ltd. pSliencer TM 4.1 CMV-FANCF-shRNA plasmid, FANCF-shRNA, and 328-microRNA fragment: were designed, synthesized and purchased by Shanghai Bioengineering Co., Ltd. Lipofectamine2000: purchased from Invitrogen Company; ExGen500: purchased from Fermentas Company; MTT assay cell viability kit, cell experiment consumables, culture medium, agarose, etc.: all purchased from Baoxin Biolo...
Embodiment 2
[0073] Implementation example 2: prepare SS-PAAs with chlorhexidine acetate monomer as guanidinylation reagent
[0074] (1) Protection of Pbf-Cl on the guanidinium structure in chlorhexidine acetate
[0075] Put 1g of chlorhexidine acetate in a 50ml three-neck bottle, add 15ml of water to dissolve, cool down to below 0°C, and adjust the pH to 11; slowly add 2g of Pbf-Cl dissolved in 10ml of acetone dropwise, and the temperature is maintained at 0°C Below, the pH is maintained in the range of 10-13; after the dropwise addition, react below 0°C for 1 hour and at room temperature for 3 hours until the pH does not change. The progress of the reaction was monitored by TLC. Suction filtration, rotary evaporation to remove acetone in the filtrate, collect the acetone phase product, oven dry at 40-60°C overnight, weigh, and obtain chlorhexidine whose guanidine structure is protected by Pbf-Cl.
[0076] (2) Synthesis of guanidinated SS-PAAs polymer with chlorhexidine as monomer
[0...
Embodiment 3
[0081] Implementation example 3: Preparation of SS-PAAs with guanidine hydrochloride monomer as guanidinylation reagent
[0082] (1) Protection of Pbf-Cl on the guanidinium structure in guanidine hydrochloride
[0083] Put 1g of guanidine hydrochloride in a 100ml three-neck flask, add 5ml of water to dissolve, cool down to below 0°C, adjust the pH to 11; slowly add 3.02g of Pbf-Cl dissolved in 10ml of acetone dropwise, and maintain the temperature at 0°C Below, the pH is maintained in the range of 10-13; after the dropwise addition, react below 0°C for 1 hour and at room temperature for 1 hour until the pH does not change. The progress of the reaction was monitored by TLC. Suction filtration, collect the precipitated product, dry in an oven at 40-60°C overnight, and weigh to obtain guanidine hydrochloride whose guanidine structure is protected by Pbf-Cl.
[0084] (2) Synthesis of guanidinated SS-PAAs polymer with guanidine hydrochloride as monomer
[0085] Take 0.19g of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com