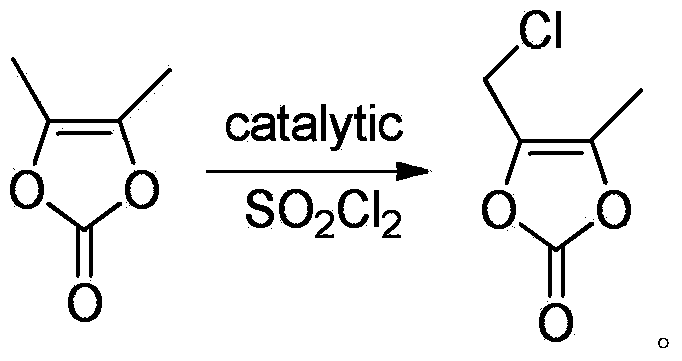
Method for catalyzed synthesis of high-purity 4-chloromethyl-5-methyl-1,3-dioxol-2-one
A technology of dioxole and synthesis method, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of reducing industrial application value, difficult to Separation, impure products and other problems, to achieve the effect of large implementation value and social and economic benefits, safe and reliable production, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Raw material and ratio
[0031] The molar ratio of the feed material is: DMDO:sulfonyl chloride is 1:1.5.
[0032] The catalyst is phenyl selenium chloride, and the molar weight of the catalyst is 5% of the molar weight of DMDO.
[0033] Organic solvent is dichloromethane, and its quality consumption is 7 times of DMDO quality.
[0034] 2. Preparation
[0035] Add 300ml of dichloromethane, 60g of DMDO and 5mol% of phenyl selenium chloride to a 500ml three-necked flask equipped with magnetic stirring, a constant pressure dropping funnel and an exhaust gas absorption device, and slowly add 106.6g of sulfonyl chloride dropwise while stirring , added dropwise for about 3 hours, reacted at room temperature for 2 hours after the dropwise addition, and removed the solvent by rotary evaporation to obtain 65.9 g of the target product with a yield of 84.4% and a purity of 98.9%.
[0036] Structure Characterization:
[0037] IR: ν max / cm -1 : 3026 (C-H), 1694 (C=C), 715 ...
Embodiment 2
[0039] 1. Raw material and ratio
[0040] The molar ratio of the feed material is: DMDO:sulfonyl chloride is 1:1.5.
[0041] The catalyst is p-methylphenyl selenium chloride, and the molar weight of the catalyst is 5% of the molar weight of DMDO.
[0042] Organic solvent is dichloromethane, and its quality consumption is 7 times of DMDO quality.
[0043] 2. Preparation
[0044] Add 300ml of dichloromethane, 60g of DMDO and 5mol% p-methylphenylselenium chloride into a 500ml three-necked flask equipped with magnetic stirring, a constant pressure dropping funnel and an exhaust gas absorption device, and slowly add 106.6 g sulfuryl chloride, added dropwise for about 3 hours, reacted at room temperature for 2 hours after the dropwise addition, and removed the solvent by rotary evaporation to obtain 53.8 g of the target product with a yield of 68.9% and a purity of 96.7%.
Embodiment 3
[0046] 1. Raw material and ratio
[0047] The molar ratio of the feed material is: DMDO:sulfonyl chloride is 1:1.5.
[0048] The catalyst is polystyrene-based selenium bromide, and the molar weight of the catalyst is 5% of the molar weight of DMDO.
[0049] Organic solvent is dichloromethane, and its quality consumption is 7 times of DMDO quality.
[0050] 2. Preparation
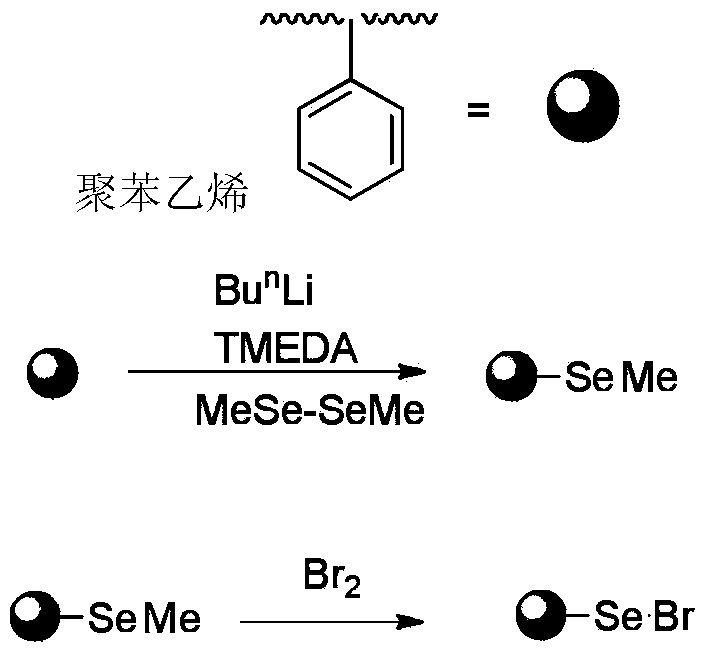
[0051] 1) Preparation of polystyrene-based selenium bromide
[0052] ① Disperse 5 grams (about 0.083 mmol) of polystyrene particles in 100 ml of cyclohexane, add 2 mmol of n-butyllithium and 2 mmol of tetramethylethylenediamine (TMEDA), react at 65 ° C for 4 hours, filter after the reaction and wash with tetrahydrofuran (THF) washing to obtain lithium-containing polystyrene;
[0053] ② Add 10.0 mmol dimethyl diselenide (MeSe-SeMe) and 100 ml THF to the lithium-containing polystyrene, and react at 0° C. for 30 min to obtain polystyrene with methyl selenium groups, which is a light yellow resin.
[0054] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com