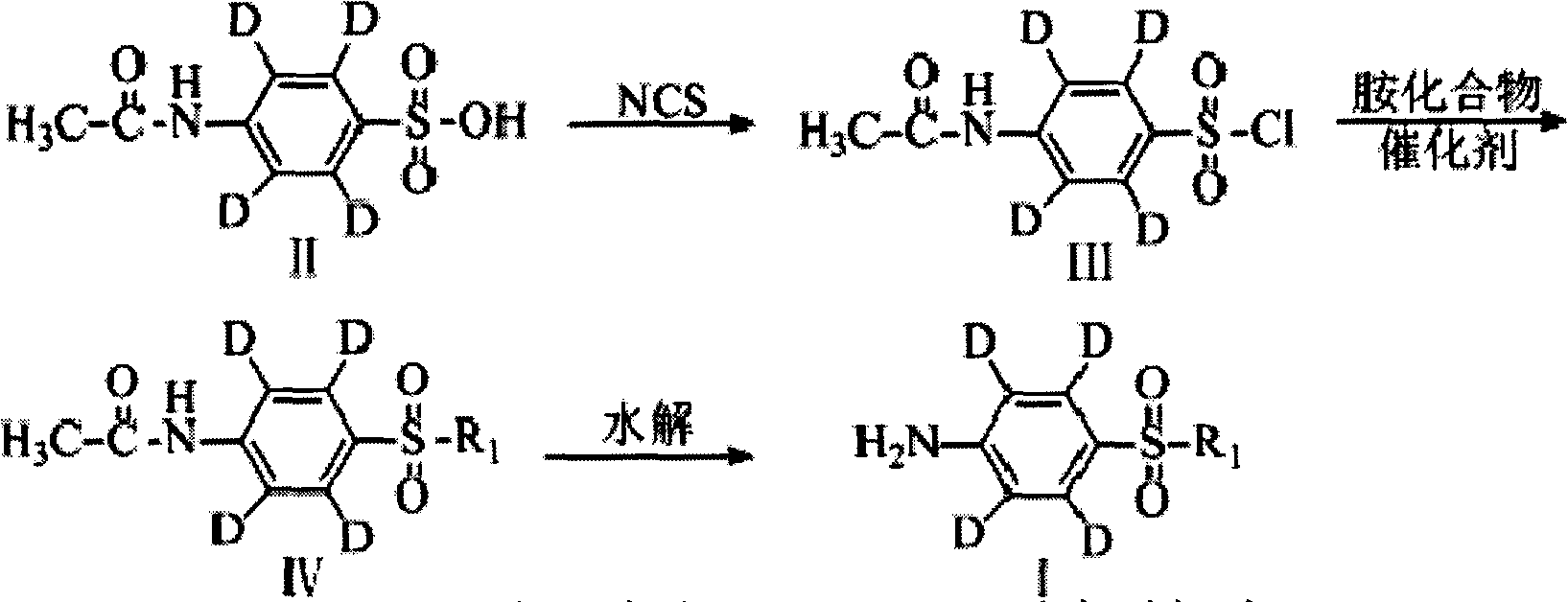
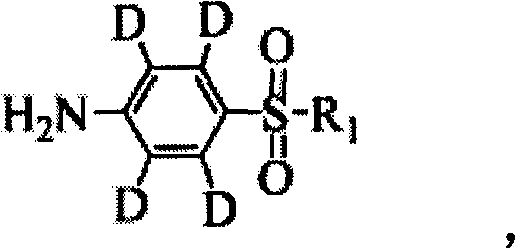
Synthetic method of deuterium-marked sulfanilamide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
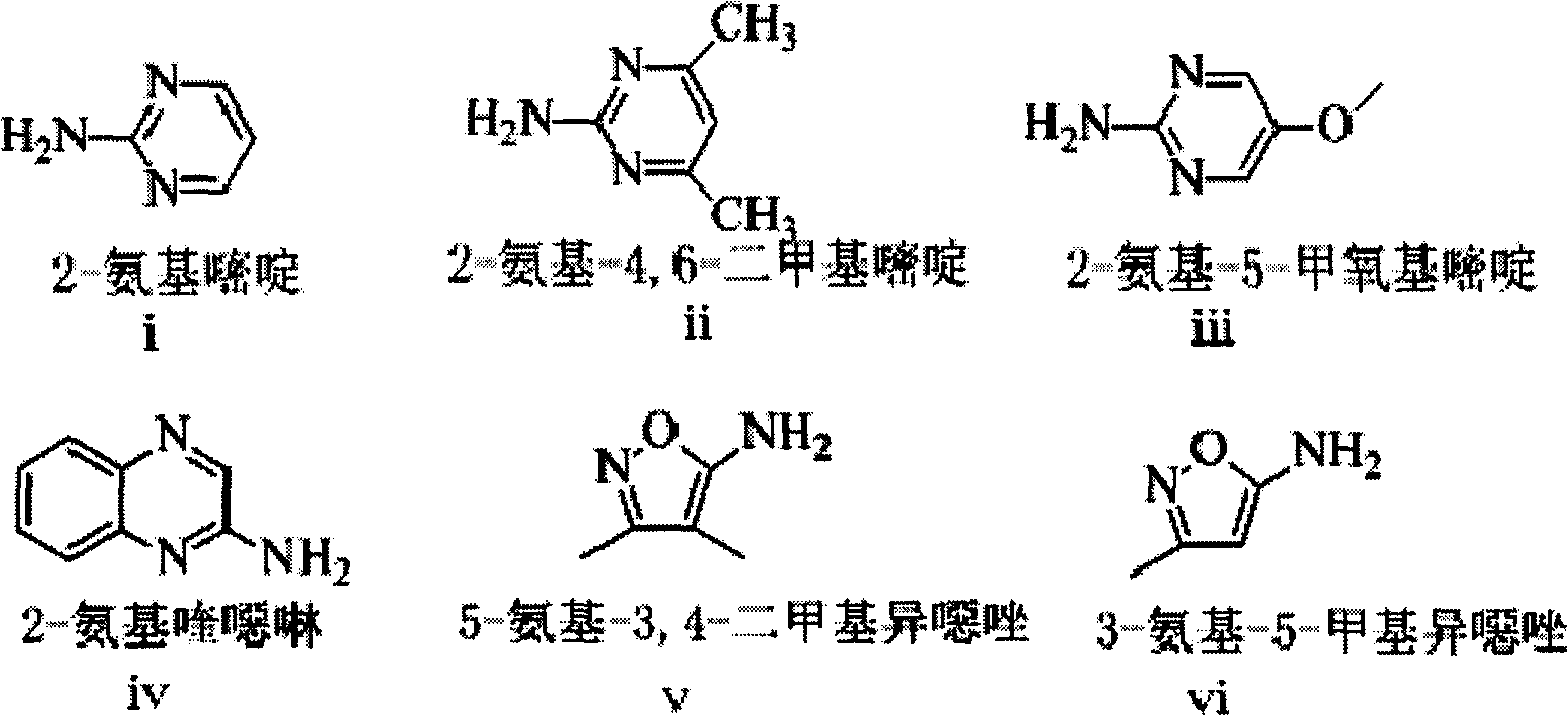
[0028] R 1 =i, sulfadiazine-benzene ring-D 4 Synthesis
[0029] acetaminobenzenesulfonic acid-benzene ring-D 4 (2.192g, 10mmol), N-chlorosuccinimide (1.600g, 12mmol), and acetone (50ml) were placed in a reaction flask, and stirred at 15-20°C for 0.5h. The mixture was cooled to 0-5°C with an ice-water bath, palladium (0.200g) was added to the reactant, and an acetone solution containing pyridine (1.58g, 20mol) and 2-aminopyrimidine (i) (1.045g, 11mmol) was added dropwise 5ml, after the dropwise addition, stir and react at 20-25°C for 2h. After the reaction, filter, add 10ml of water to the filtrate, neutralize the pH to 5-6 with 1N hydrochloric acid, wash twice with 50ml of water, dry with a desiccant, filter, and concentrate the filtrate to dryness under reduced pressure. Put the obtained solid in a reaction bottle, add 5ml of 30% sodium hydroxide, react at 60°C for 15min, cool the reaction system to 0-5°C in an ice-water bath, add 1N hydrochloric acid to neutralize the pH...
Embodiment 2
[0031] R 1 =ii, sulfamethazine-benzene ring-D 4 Synthesis
[0032]P-acetaminobenzenesulfonic acid (2.192g, 10mmol), N-chlorosuccinimide (1.600g, 12mmol), and acetone (50ml) were placed in a reaction flask, and stirred at 20-25°C for 1h. The mixture was cooled to 0-5°C with an ice-water bath, iridium (0.200g) was added to the reactant, and a mixture containing pyridine (1.580g, 20mol), 2-amino-4,6-dimethylpyrimidine (ii) was added dropwise ( 1.353g, 11mmol) of acetone solution in 5ml, after the dropwise addition was completed, the reaction was stirred at 20-25°C for 2.5h. After the reaction, filter, add 10ml of water to the filtrate, neutralize the pH to 5-6 with 1N hydrochloric acid, wash twice with 50ml of water, dry with a desiccant, filter, and concentrate the filtrate to dryness under reduced pressure. Put the obtained solid in a reaction flask, add 5ml of 30% sodium hydroxide, react at 60°C for 15 minutes, cool the reaction system to 0-5°C in an ice-water bath, add 1N ...
Embodiment 3
[0034] R 1 =iii, sulfamethoxine-benzene ring-D 4 Synthesis
[0035] acetaminobenzenesulfonic acid-benzene ring-D 4 (2.192g, 10mmol), N-chlorosuccinimide (1.600g, 12mmol), and acetone (50ml) were placed in a reaction flask, and stirred at 10-15°C for 1h. Cool the mixture to 0-5°C with an ice-water bath, add indium (0.100g) to the reactant, and dropwise add pyridine (1.580g, 20mol), 2-amino-5-methoxypyrimidine (iii) (1.375g , 11mmol) of acetone solution in 5ml, after the dropwise addition was completed, the reaction was stirred at 20-25°C for 2h. After the reaction, filter, add 10ml of water to the filtrate, neutralize the pH to 5-6 with 1N hydrochloric acid, wash twice with 50ml of water, dry with a desiccant, filter, and concentrate the filtrate to dryness under reduced pressure. Put the obtained solid in a reaction flask, add 5ml of 30% sodium hydroxide, react at 60°C for 20 minutes, cool the reaction system to 0-5°C in an ice-water bath, add 1N hydrochloric acid to neutr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com