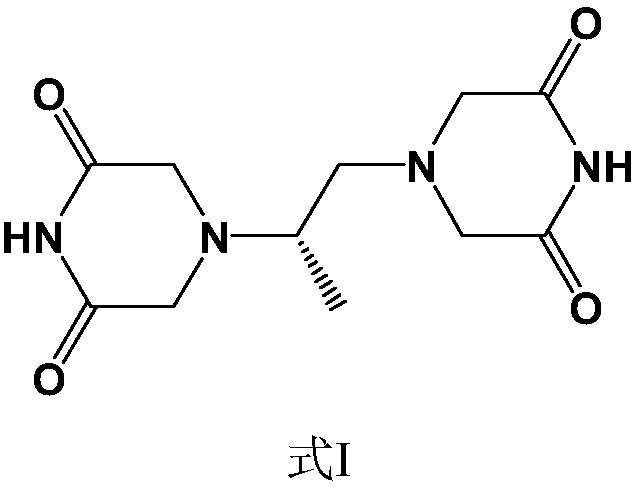
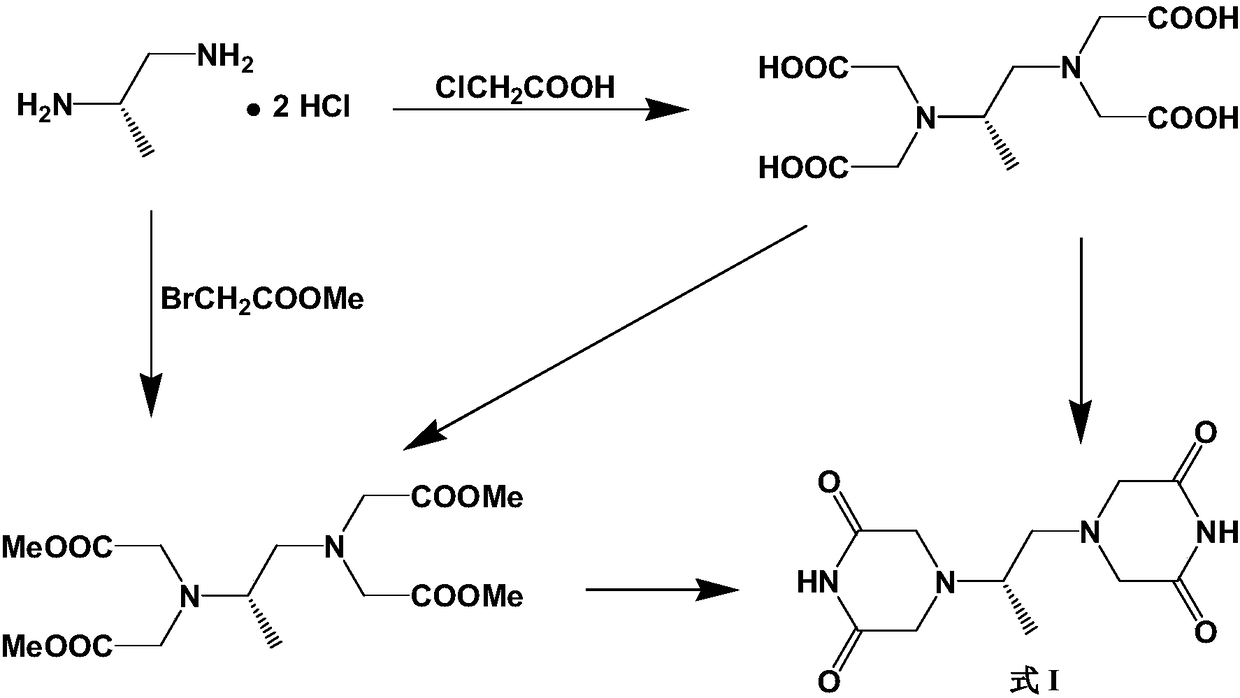
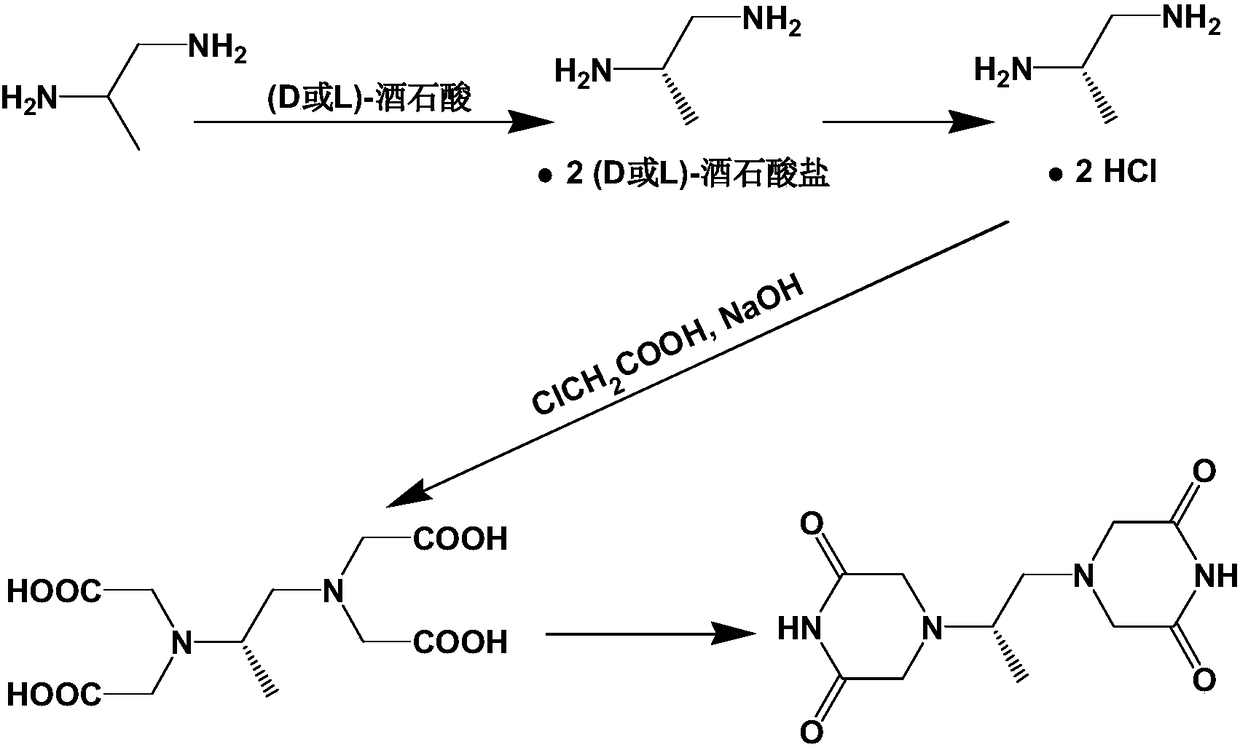
Preparation method of piperazine diketone type compound
A compound and preparation process technology, applied in the field of drug synthesis, can solve problems such as long reaction time, uneconomical environmental protection, environmental pollution, etc., and achieve high yield, cost reduction, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of (S)-1,2-diaminopropane-tetraacetic acid methyl ester
[0044] Chloroacetic acid (192.5 g), (S)-1,2-diaminopropane bis-D-tartrate (50 g) and water (400 mL) were added to a round bottom flask, NaOH (125 g) / water (210 mL) was added dropwise, Stir at 45°C for 96h. The reaction solution was concentrated to dryness to obtain a viscous substance, which was impregnated with methanol (1000 mL), filtered, and the filter cake was washed with methanol. The combined filtrates were concentrated under reduced pressure. The residue was dissolved in methanol (1000 mL), concentrated sulfuric acid (50 mL) was added dropwise, and refluxed for 36 h. Cool down to room temperature, add sodium carbonate (70g), adjust pH = 9, distill under reduced pressure to remove methanol, extract the residual solution 3 times with ethyl acetate, combine the extracts, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain a colorless li...
Embodiment 2
[0046] Embodiment 2: the preparation of (S)-1,2-diaminopropane-tetraacetic acid methyl ester
[0047] Chloroacetic acid (115.5 g), (S)-1,2-diaminopropane bis-L-tartrate (30 g) and water (250 mL) were added to a round bottom flask, NaOH (75 g) / water (125 mL) was added dropwise, Stir at 45°C for 96h. The reaction solution was concentrated to dryness to obtain a viscous substance, which was impregnated with methanol (600 mL), filtered, and the filter cake was washed with methanol. The combined filtrates were concentrated under reduced pressure. The residue was dissolved in methanol (600 mL), concentrated sulfuric acid (30 mL) was added dropwise, and refluxed for 36 h. Cool down to room temperature, add sodium carbonate (42g), adjust pH = 9, distill off methanol under reduced pressure, extract the residual solution 3 times with ethyl acetate, combine the extracts, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain a colorless liquid 34...
Embodiment 3
[0048] Embodiment 3: the preparation of (S)-1,2-diaminopropane-tetraacetic acid methyl ester
[0049]Add bromoacetic acid (170g), (S)-1,2-diaminopropane bis-D-tartrate (30g) and water (250mL) to a round bottom flask, add NaOH (75g) / water (125mL) dropwise, 45 Stir at ℃ for 96h. The reaction solution was concentrated to dryness to obtain a viscous substance, which was impregnated with methanol (600 mL), filtered, and the filter cake was washed with methanol. The combined filtrates were concentrated under reduced pressure. The residue was dissolved in methanol (600 mL), concentrated sulfuric acid (30 mL) was added dropwise, and refluxed for 36 h. Cool down to room temperature, add sodium carbonate (42g), adjust pH = 9, distill off methanol under reduced pressure, extract the residual solution 3 times with ethyl acetate, combine the extracts, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain a colorless liquid 35.7g, product yield 73....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com