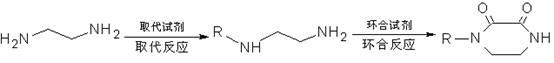
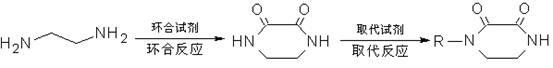
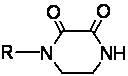
Method for synthesizing N-monosubstituted piperazine-2,3-dione
A synthetic method and mono-substitution technology, which is applied in the field of synthesis of N-monosubstituted piperazine-2,3-dione, can solve the problems of many by-products, high energy consumption for separation, and low production efficiency, and achieve stable product quality , post-processing is simple, the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Take 30.0g of ethylenediamine, add 150ml of methanol, mix well and put it in the dropping funnel I; take 65.0g of dimethyl oxalate, add 400ml of methanol, dissolve it and put it in the dropping funnel II; take another 150ml of methanol and put it in the dropping funnel In a 1000ml reactor, lower the temperature to 0-5°C, and at the same time add dropwise methanol dilutions of ethylenediamine and dimethyl oxalate for about 1.5-2 hours. Then raise the temperature to 40-45°C, stir and react for 3.5-4 hours. After the reaction is completed, adopt the method of concentration under reduced pressure first under normal pressure, distill about 500ml of methanol, cool down, stand still, filter, wash, and dry to obtain 55.4g of white intermediate product piperazine-2,3-dione (yield 97.19 %, purity 98.82%; FT-IR σ / cm -1 : 3302, 3064, 2951, 1641, 1521, 1452, 1373, 1317, 1249, 1220, 827, 742, 540; 1 H NMR (600MHz, DMSO) δ / ppm: 3.164-3.172 (s, 4H, CH 2 -CH 2 ), 7.324 (s, 2H, N-H))...
Embodiment 2
[0040] Take 30.0g of ethylenediamine, add 150ml of ethanol, mix well and put it in the dropping funnel I; take 76.5g of diethyl oxalate, add 450ml of ethanol, mix it and put it in the dropping funnel II; take another 150ml of ethanol and put it in the dropping funnel In a 1000ml reactor, lower the temperature to 5-10°C, and at the same time add the ethanol dilution of ethylenediamine and diethyl oxalate dropwise, and drop it in about 1.5-2 hours. Then raise the temperature to 45-50°C, stir and react for 4-4.5 hours. After the reaction was completed, the method of concentration under reduced pressure was adopted first, and about 300ml of ethanol was evaporated, and the remaining reaction solution was directly used for the next step of substitution reaction.
[0041] Take the remaining reaction solution from the previous step, slowly add 84.4g of 48% hydrobromic acid dropwise under stirring, stir at room temperature for 1 hour, then raise the temperature to 38-40°C, slowly add 5...
Embodiment 3
[0043] Take 30.0g of ethylenediamine, add 150ml of ethanol, mix well and put it in the dropping funnel I; take 76.5g of diethyl oxalate, add 400ml of ethanol, mix it and put it in the dropping funnel II; take another 150ml of ethanol and put it in the dropping funnel In a 1000ml reactor, lower the temperature to 5-10°C, and at the same time add the ethanol dilution of ethylenediamine and diethyl oxalate dropwise, and drop it in about 1.5-2 hours. Then raise the temperature to 45-50°C, stir and react for 4-4.5 hours. After the reaction is completed, adopt the method of concentrating under reduced pressure first under normal pressure, distill out about 500ml of ethanol, cool down, stand still, filter, wash, and dry to obtain 56.4g of white intermediate product piperazine-2,3-dione (yield 98.95 %, purity 99.38%). Combine the filtrate and washing solution, and recover the sleeve for the next batch of cyclization reactions.
[0044] Dissolve 50.0g of piperazine-2,3-dione (purity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com