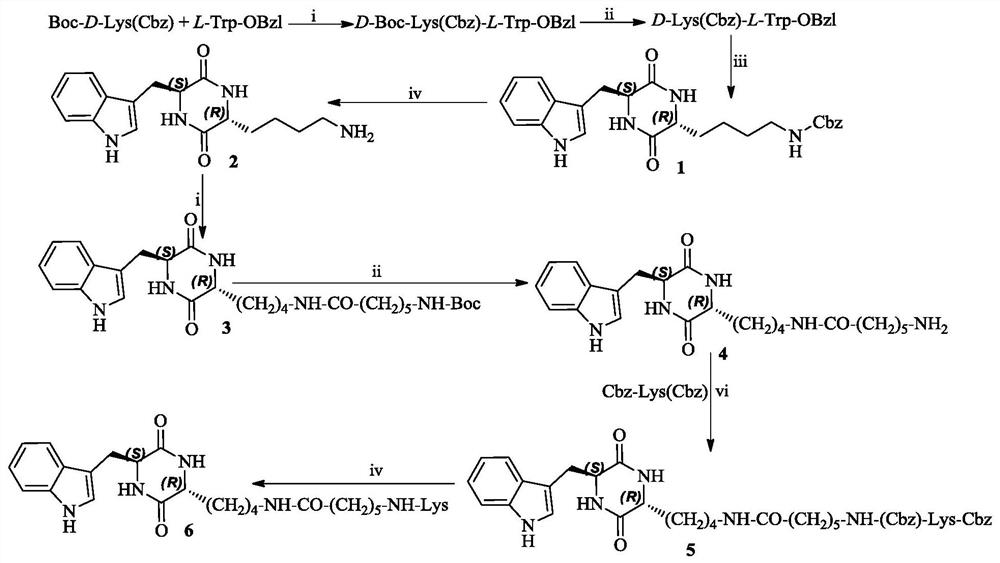
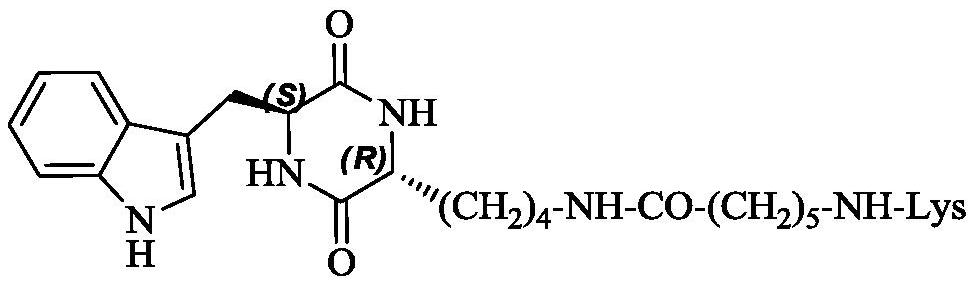
3r-indolemethyl-6s-lys modified piperazine-2,5-dione, its synthesis, activity and application
A technology of -l-trp-obzl and indole, applied in drug combination, non-central analgesics, organic chemistry, etc., can solve the problems of no anti-tumor metastasis effect and unsatisfactory clinical curative effect of tumor chemotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 prepares D-Boc-Lys(Cbz)-L-Trp-OBzl
[0021] Add 0.68 g (5.0 mmol) 1-hydroxybenzotriazole (HOBt) and 1.24 g (6.0 mmol) dicyclohexylcarbodiimide (DCC) and stirred for 30 minutes to obtain reaction solution A. 1.47g (5.0mmol) L-Trp-OBzl was dissolved in 20mL dry THF, and N-methylmorpholine (NMM) was added to adjust the pH to 9 to obtain reaction solution B. The reaction solution B was added into the reaction solution A, and reacted at room temperature for 12 hours. TLC (dichloromethane / methanol, 40 / 1) showed that the reaction was complete. The reaction mixture was filtered, the filtrate was concentrated under reduced pressure, and the residue was dissolved in 50 mL of ethyl acetate. The resulting solution was sequentially washed with saturated NaHCO 3 Wash with aqueous solution (25mL×3), wash with saturated NaCl aqueous solution (25mL×3), 5% KHSO 4 Wash with aqueous solution (25mL×3), wash with saturated NaCl aqueous solution (25mL×3), and wash with satura...
Embodiment 2
[0022] Embodiment 2 prepares D-Lys(Cbz)-L-Trp-OBzl
[0023] To 2.62g (4.0mmol) D-Boc-Lys(Cbz)-L-Trp-OBzl under ice-cooling, slowly add 30mL of hydrogen chloride in ethyl acetate solution (4M) and stir for 4 hours, TLC (dichloromethane / methanol, 40 / 1) shows that the reaction is complete. The reaction mixture was concentrated under reduced pressure, and the residue was dissolved in 30 mL of anhydrous ethyl acetate. The obtained solution was concentrated under reduced pressure, and the residue was dissolved in 30 mL of anhydrous ethyl acetate. This operation was repeated three times. Add 30 mL of anhydrous diethyl ether to the residue to suspend by means of a sonicator, and remove the diethyl ether after standing still to obtain 2.07 g (93%) of the title compound as a yellow powder. ESI-MS(m / z):557[M+H] + .
Embodiment 3
[0024] Example 3 Preparation of (3R,6S)-3-(benzyloxycarbonylamino-n-butyl)-6-(indole-3-methyl)-piperazine-2,5-dione (1)
[0025] A solution of 1.95g (3.5mmol) D-Lys(Cbz)-L-Trp-OBzl and 50mL ethyl acetate was washed with saturated NaHCO 3 After fully washing with aqueous solution (25 mL×3), the ethyl acetate layer was stirred at 80° C. for 56 hours. TLC (dichloromethane / methanol, 20 / 1) showed the reaction was complete. The reaction mixture was allowed to stand well at room temperature and filtered to afford 0.72 g (46%) of the title compound as a colorless solid. ESI-MS(m / z):449[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com