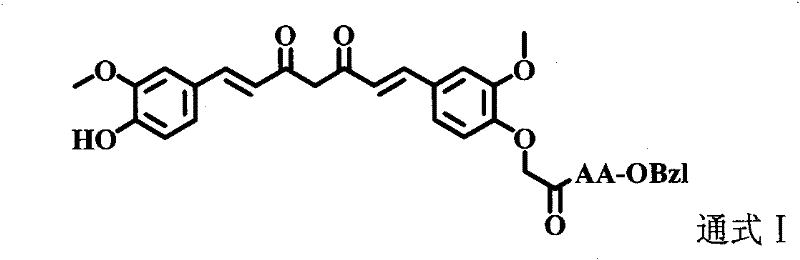
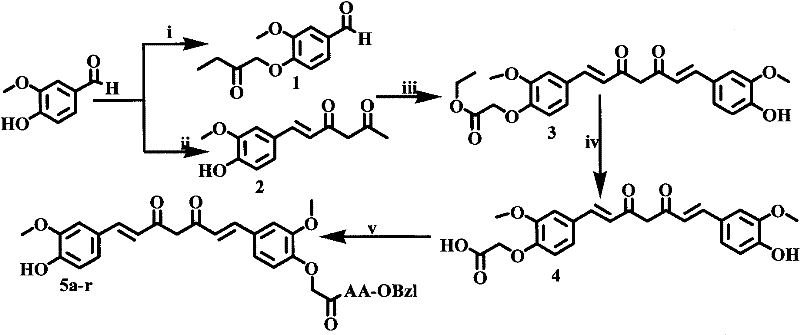
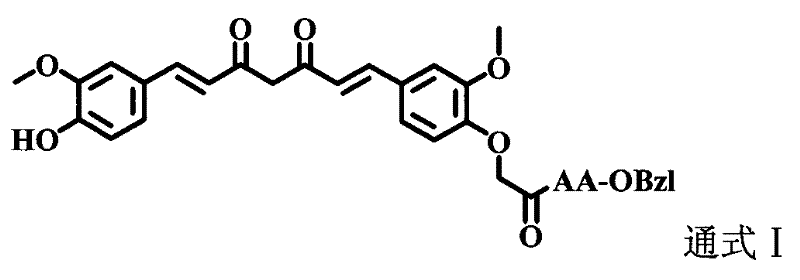
Amino acid modified curcumin, synthesis method thereof, and application thereof
A technology of oxyacetyl amino acid benzyl ester group and seryl group, which is applied in the field of curcumin derivatives, can solve the problems of application limitations, low absorption in the body, and excessive metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 prepares 3-methoxy-4-(2-butyryloxy) benzaldehyde (1)
[0022] 10 g (65.7 mmol) of vanillin were dissolved in 200 ml of anhydrous THF. To the resulting solution, 11.8 g (85.6 mmol) of potassium carbonate was added in portions and stirred for 2 h. Then 7.68ml of ethyl bromoacetate was added dropwise, and stirred vigorously at room temperature for 48h. TLC plate monitoring (petroleum ether:ethyl acetate=3:1) showed that the reaction was complete. The reaction mixture was suction filtered, and the filtrate was concentrated to dryness under reduced pressure. The obtained residue was dissolved in 150 mL of ethyl acetate, placed in a 250 mL separatory funnel, washed successively with 5% potassium hydrogensulfate aqueous solution (30 mL×3), and saturated sodium chloride aqueous solution (30 mL×3). The ethyl acetate layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 10.9 g (70%) of...
Embodiment 2
[0023] Example 2 Preparation of 6-(4-hydroxyl-3-methoxyphenyl)-5,6-hexene-2,4-dione (2)
[0024] Add 10.58ml (102.9mmol) of acetylacetone, 5.0g (72.1mmol) of boron oxide and 50.0mL of ethyl acetate into a 150mL three-necked flask, install a reflux condenser, and stir for 1 hour at a constant temperature of 70°C in an oil bath. Then 5.2 g (34.3 mmol) of vanillin and 4.8 ml (34.3 mmol) of tributyl borate were added. The reaction mixture was stirred at 85 °C for 30 min. 1.8 mL (41.1 mmol) of triethylamine diluted in 10 mL of ethyl acetate was slowly added dropwise to the three-necked flask within 30 min, reacted at 100° C. for 1 h, and then cooled to room temperature. Add 36mL of hydrochloric acid (1N) dropwise to the reaction flask, stir at 50°C for 30min, let stand, and fully separate the layers. The aqueous layer was extracted 3 times with ethyl acetate, the ethyl acetate layers were combined, washed with water until neutral, dried over anhydrous sodium sulfate, filtered, th...
Embodiment 3
[0025] Example 3 Preparation of 1-(4-hydroxyl-3-methoxyphenyl)-7-(4-oxyacetoethoxy-3-methoxyphenyl)-1,6-heptadiene-3, 5-diketone(3)
[0026]Add 5.0g (21.4mmol) 6-(4-hydroxyl-3-methoxyphenyl)-5,6-hexene-2,4-dione (2), 1.0g (14.9mmol ) boron oxide and 50.0 mL ethyl acetate. Install the reflux condenser. The reaction mixture was stirred at 70 °C for 1 h. Then 4.3 g (18.1 mmol) of 3-methoxy-4-(2-butyryloxy)benzaldehyde (1) and 3.7 ml (21.4 mmol) of tributyl borate were added. The reaction mixture was stirred at 85 °C for 30 min. 0.98 mL (21.4 mmol) of n-butylamine diluted in 10 mL of ethyl acetate was slowly added dropwise to the three-necked flask within 30 min. The reaction mixture was continued at 100 °C for 1 h, then cooled to room temperature. Add 19.6 mL of hydrochloric acid (1N) to the reaction mixture, stir the reaction at 50°C for 30 min, let it stand still, separate the layers sufficiently, and extract the aqueous layer with ethyl acetate three times. The combined...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com