Polymorphism of deuterium-substituted plinabulin compound and preparation method and application of polymorphism
A technology of deuterated methylene and crystal form, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
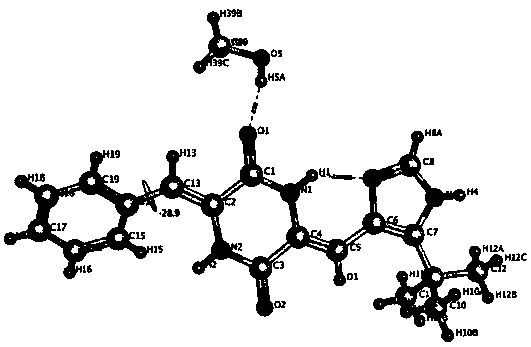
[0052] Preparation of (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)deuteromethylene)piperazine-2,5-dione
[0053] Its specific preparation process comprises the following steps:
[0054] 1) Preparation of ethyl 5-(tert-butyl)oxazole-4-carboxylate
[0055] Add 90g (796mmol) ethyl isocyanoacetate to 1000mL tetrahydrofuran, slowly dropwise add 145g (955mmol) DBU, then dropwise add 178g (955mmol) trimethylacetic anhydride, and stir the reaction at room temperature for 48h after dropping. After the reaction was completed, it was concentrated under reduced pressure. For extraction, add 1500mL of dichloromethane, wash with 800mL of 10% sodium carbonate, 800mL of 10% citric acid, and 800mL of saturated brine, and back-extract the aqueous phase twice with 1000mL of dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate, filtered with suction after half an hour, and concentrated under reduced pressure. After passing through a silica gel (200...
Embodiment 2
[0073] (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)deuterated methylene)piperazine-2,5-dione purification process and Preparation of α crystal form
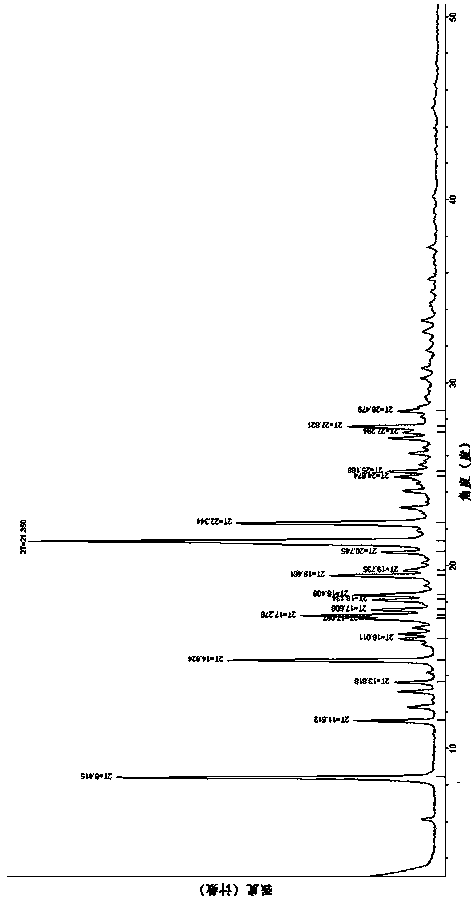
[0074] 6.66 g of (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)deuteromethylene)piperazine-2,5 - Place the crude diketone in a brown bottle, add 400mL of isopropanol under heating conditions until it is completely dissolved, then add 160mL of water without crystallization, place at room temperature, stir and cool to crystallize, filter with suction, isopropanol: water = Wash the filter cake at a ratio of 1:1, beat the filter cake with 100 mL of ethyl acetate for 10 h, filter, wash the filter cake with ethyl acetate, and dry to obtain 5.323 g of a yellow powdery solid. The obtained solid crystal form is α crystal form, and the yield is 80.0%. . The main characteristic peaks of the X-ray powder 2θ angle diffraction peaks of the α crystal form are shown in Table 1, and the specific diffraction patterns are sh...
Embodiment 3
[0080] (3Z,6Z)-3-Benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)deuteromethylene)piperazine-2,5-dione β crystal form preparation
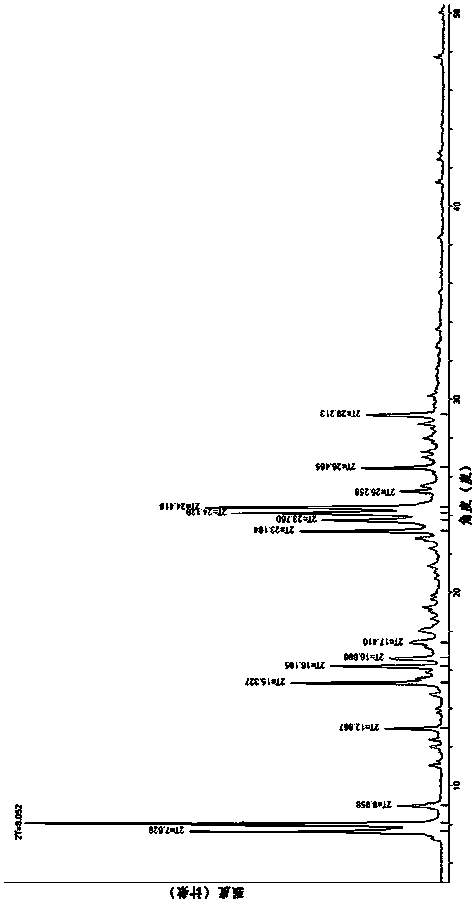
[0081] The specific preparation process includes the following steps: Weigh (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl) deuterated methylene) piperazine-2 , 5-diketone (200mg, 0.59mmol), using a mixed solution of 20mL methanol and 0.1mL water as a solvent, dissolved at 70°C, filtered into a crystallization dish, covered the bottle mouth of the crystallization dish with plastic wrap, and placed on the plastic wrap Prick 16 holes with a capillary tube with an outer diameter of 0.5mm, and place it in the dark at 25°C for evaporation. After 72 hours, β-crystal crystals are precipitated, filtered, and dried to obtain 142 mg of a cubic solid with a yield of 71%. The obtained β-crystal The melting point of the type is 263.6-264.4°C. The obtained β crystal form is tested by X-ray powder diffraction, and the characteristic peaks of 2θ diffract...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com