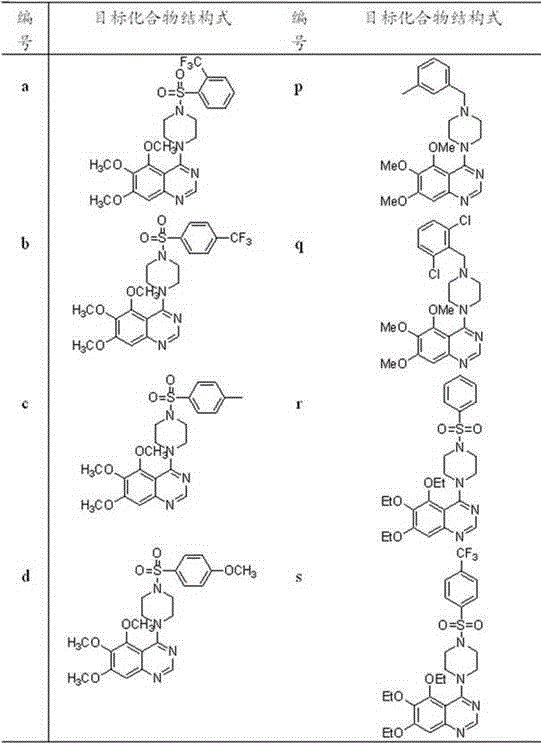
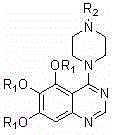
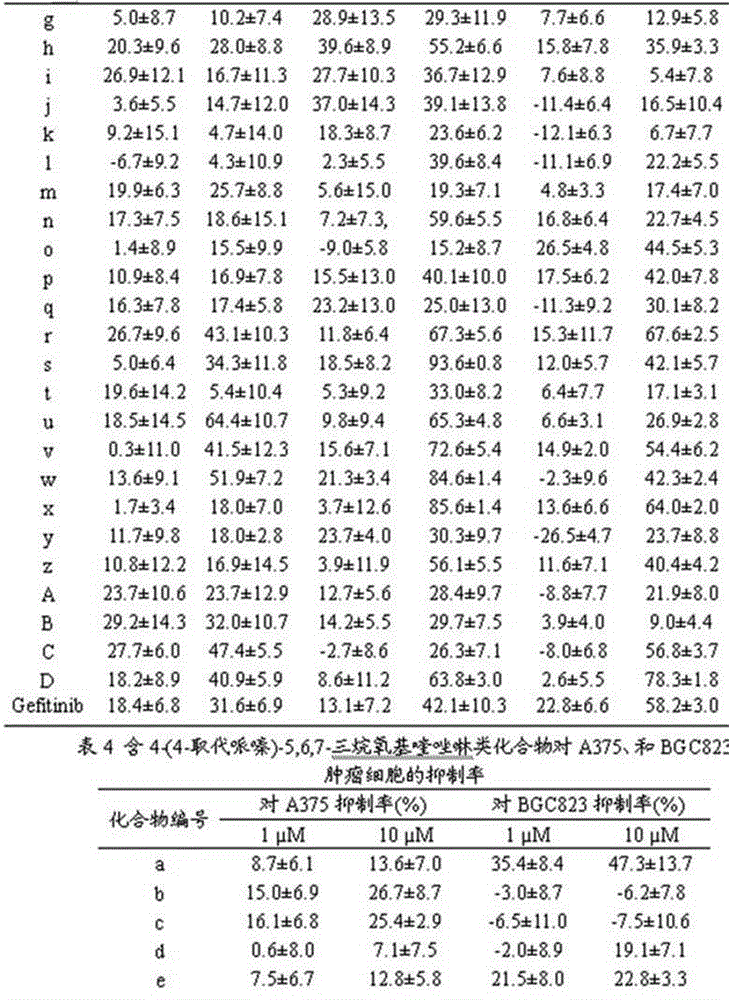
4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound as well as preparation method and application of 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound
A trialkoxyquinazoline and compound technology, which is applied in the field of preparation of 4--5,6,7-trialkoxyquinazoline compounds, can solve the problems of anti-phytopathogenic fungi and low anti-cancer activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1, 5,6,7-trimethoxy-4-(4-(2-(trifluoromethyl)benzenesulfonyl)piperazine)quinazoline
[0105] (Compound number is a ) Synthesis
[0106] (1) Synthesis of 2,3,4-trimethoxybenzoic acid
[0107] Stir 5.0 g of 2,3,4-trihydroxybenzoic acid and 20 mL of water in a bottle, and add 25 mL of sodium hydroxide solution (4mol / L) and 20 g of dimethyl sulfate dropwise in a water bath at room temperature. After the dropwise addition, heat to reflux for 6 h, stop the reaction, use hydrochloric acid to adjust the PH value to neutral, a brown solid precipitates out, filter and dry the crude product to obtain the crude product, extract the filtrate with chloroform to obtain the crude product, combine the crude product with column layer The target compound was separated and purified by analysis to obtain 4.2 g of a white solid with a yield of 67.3%.
[0108] (2) Synthesis of methyl 2,3,4-trimethoxybenzoate
[0109] Mix and stir 1.2 g of 2,3,4-trimethoxybenzoic acid and 5 mL of m...
Embodiment 2
[0124] Example 2. Compound 5,6,7-trimethoxy-4-(4-(4-methylbenzenesulfonyl)piperazine)quinazoline
[0125] (Compound number is c )Synthesis
[0126] (1) Synthesis of 2,3,4-trimethoxybenzoic acid
[0127] Synthesize as embodiment one (1) condition and method
[0128] (2) Synthesis of methyl 2,3,4-trimethoxybenzoate
[0129] Synthesize as embodiment one (2) condition and method
[0130] (3) Synthesis of methyl 6-nitro-2,3,4-trimethoxybenzoate
[0131] Synthesize as embodiment one (3) condition and method
[0132] (4) Synthesis of methyl 6-amino-2,3,4-trimethoxybenzoate
[0133] Synthesize as embodiment one (4) condition and method
[0134] (5) 5,6,7-trimethoxyquinazoline-4-(3H )-ketone synthesis
[0135] Synthesize as embodiment one (5) condition and method
[0136] (6) Synthesis of 4-chloro-5,6,7-trimethoxyquinazoline
[0137] Synthesize as embodiment one (6) condition and method
[0138] (7) Synthesis of 4-(N-Boc-piperazine)-5,6,7-trimethoxyquinazoline
[0139] Syn...
Embodiment 3
[0144] Embodiment three, compound 5,6,7-trimethoxy-4-(4-(4-methoxybenzenesulfonyl) piperazine) quinazoline (the compound number is d )Synthesis
[0145] (1) Synthesis of 2,3,4-trimethoxybenzoic acid
[0146] Synthesize as embodiment one (1) condition and method
[0147] (2) Synthesis of methyl 2,3,4-trimethoxybenzoate
[0148] Synthesize as embodiment one (2) condition and method
[0149] (3) Synthesis of methyl 6-nitro-2,3,4-trimethoxybenzoate
[0150] Synthesize as embodiment one (3) condition and method
[0151] (4) Synthesis of methyl 6-amino-2,3,4-trimethoxybenzoate
[0152] Synthesize as embodiment one (4) condition and method
[0153] (5) 5,6,7-trimethoxyquinazoline-4-( 3H )-ketone synthesis
[0154] Synthesize as embodiment one (5) condition and method
[0155] (6) Synthesis of 4-chloro-5,6,7-trimethoxyquinazoline
[0156] Synthesize as embodiment one (6) condition and method
[0157] (7) Synthesis of 4-(N-Boc-piperazine)-5,6,7-trimethoxyquinazoline
[015...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com