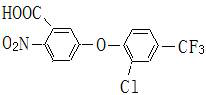
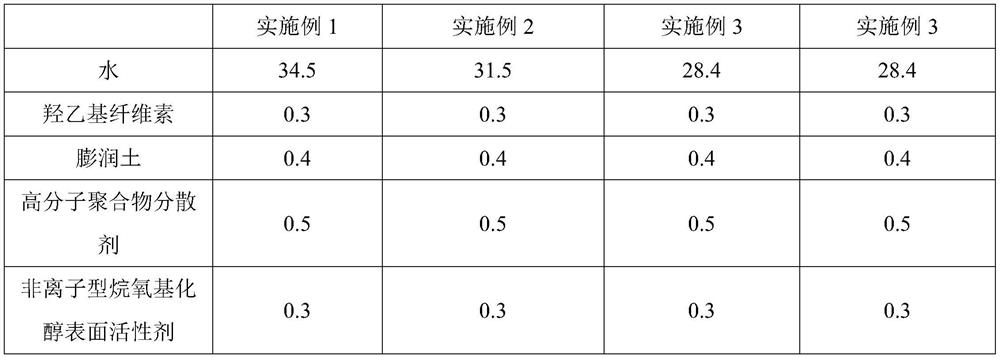
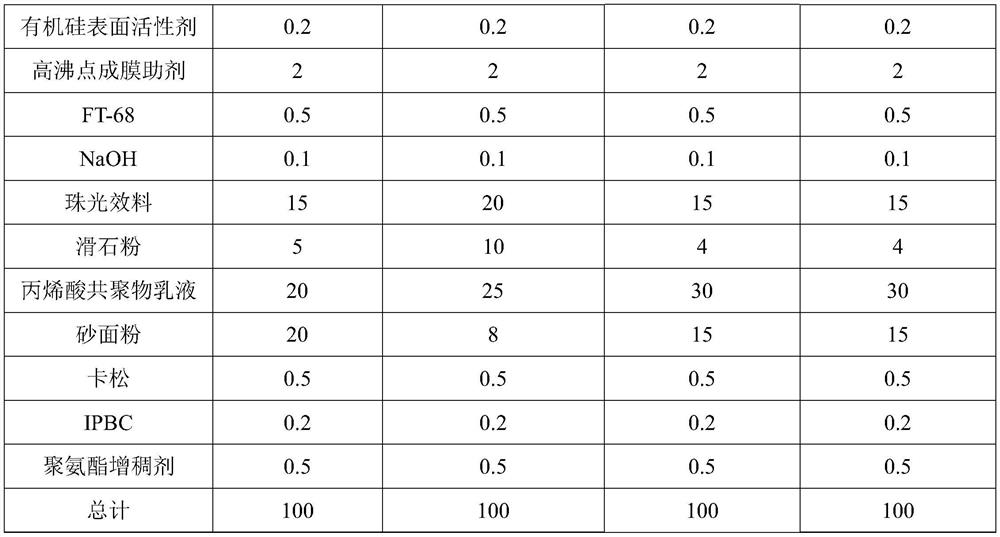
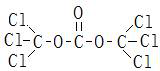Patents
Literature
48 results about "Chlorethoxyfos" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chlorethoxyfos (O,O-diethyl-O-(1,2,2,2-tetrachloroethyl)phosphorothioate) is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is registered for the control of corn rootworms, wireworms, cutworms, seed corn maggot, white grubs and symphylans on corn. The insecticide is sold under the trade name Fortress by E.I. du Pont de Nemours & Company.
Preparation method for 4-methyl-5-ethyoxyl-oxazole
InactiveCN102321043AEasy to operateEase of industrial productionOrganic chemistryChlorethoxyfosOrganic layer
The invention relates to a preparation method for 4-methyl-5-ethyoxyl-oxazole. The preparation method for the 4-methyl-5-ethyoxyl-oxazole comprises the following steps of: performing cyclization reaction on N-ethoxyl oxalyl alanine ethyl ester under the action of phosphorus oxychloride / triethylamine / dimethylformamide used as a cyclization dehydrating agent at the temperature of between 40 and 60 DEG C for 0.5 to 1 hour; heating to 75 to 100 DEG C and reacting for 5 to 10 hours; hydrolyzing the reaction materials and separating out a water layer; adding an aqueous solution of sodium hydroxide into an organic layer to hydrolyze, adjusting the pH value to be 12 to 14, distilling to obtain ethanol and adjusting the pH value to be 2.0 to 3.0 by adding sulfuric acid; heating to 65 DEG C to perform decarboxylation; adjusting the pH value to 8.0 to 10.0 by using alkali; after chloroform extraction, drying the organic layer by using anhydrous sodium sulfate; and distilling the chloroform under normal pressure to obtain the target product 4-methyl-5-ethyoxyl-oxazole. The process is easy to operate and by the preparation method, industrialized production is easy to realize; the reaction condition is mild, side reaction is few, reaction yield is high and the product content is high; and toxic methylbenzene is not used, so physical health of staff and environmental protection are facilitated.
Owner:HUBEI HUISHENG PHARMA
Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine
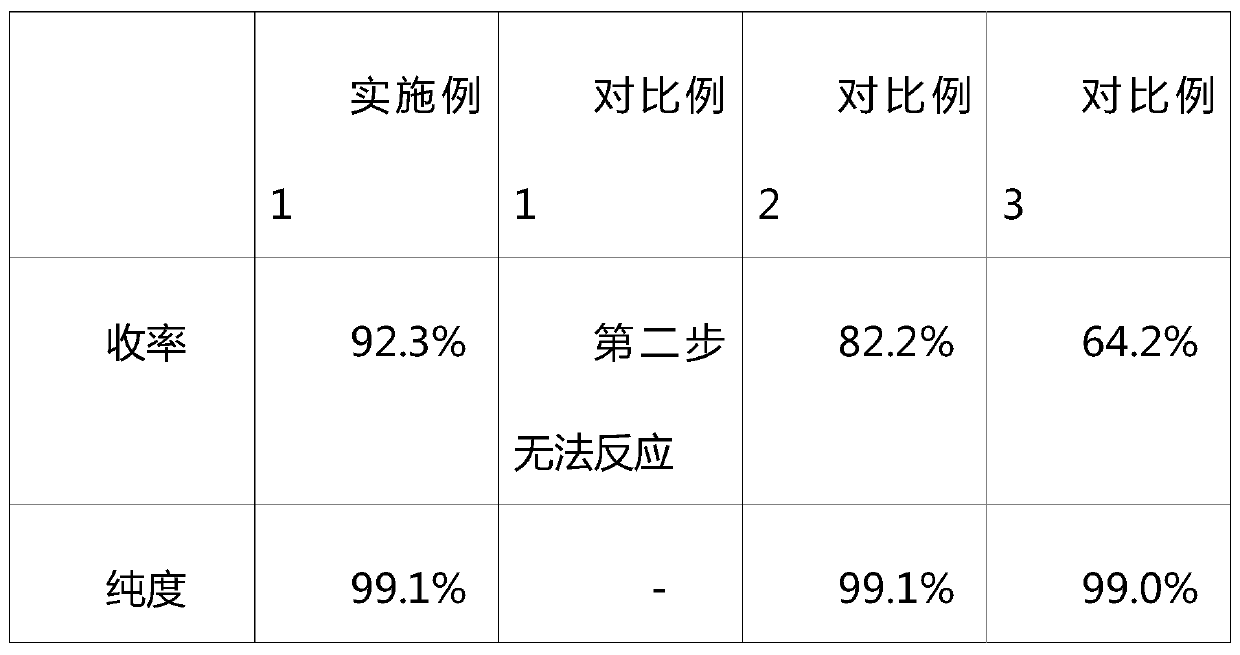
The invention relates to a synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine. The invention solves the problems such as low yield, high pollution, complicated operation, and the like of current preparation methods. The method provided by the invention comprises the steps that: methoxy methyl acetate and methyl formate are condensed under a strong-alkali condition; the condensation product is subjected to cyclization with thiourea; methylation is carried out by using chloromethane; chlorination is carried out by using phosphorus oxychloride; hydrazination is carried out by using hydrazine hydrate; the product is subjected to cyclization with cyano bromine; and under the effect of strong alkali and acrylate, a final product is obtained. With the method provided by the invention, the yield of each step is higher than 80%, and a total yield reaches 39%. The method is suitable for industrialized productions. The method provided by the invention belongs to the field of paddy rice herbicide penoxsulam intermediate preparation.
Owner:HEILONGJIANG UNIV
A flame retardant and its synthetic process
InactiveCN102277176ANo pollution in the processGood synergyFibre treatmentPhosphorus organic compoundsPolymer scienceFire retardant
The invention discloses a chlorinated alkyl polyphosphate flame retardant and a synthesis process thereof. The flame retardant is an intermediate formed by reacting phosphorus oxychloride and diethylene glycol as raw materials, and the intermediate is then combined with epoxy Propane reacts under the condition of catalyst, synthesizes and obtains the product of the present invention; The advantage of the present invention is: technique is simple, and reaction process is easy to control, and the synthetic chloroalkyl polyphosphate is mainly used as rubber, polyurethane, artificial fiber, artificial leather etc. The flame retardant of the material has stable flame retardant performance. It can be used as a flame retardant, a stabilizer, a flame retardant plasticizer, and a crosslinking agent.
Owner:SHOUGUANG WEIDONG CHEM
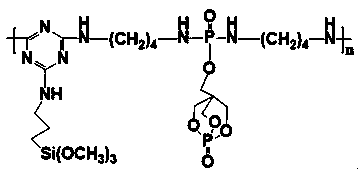
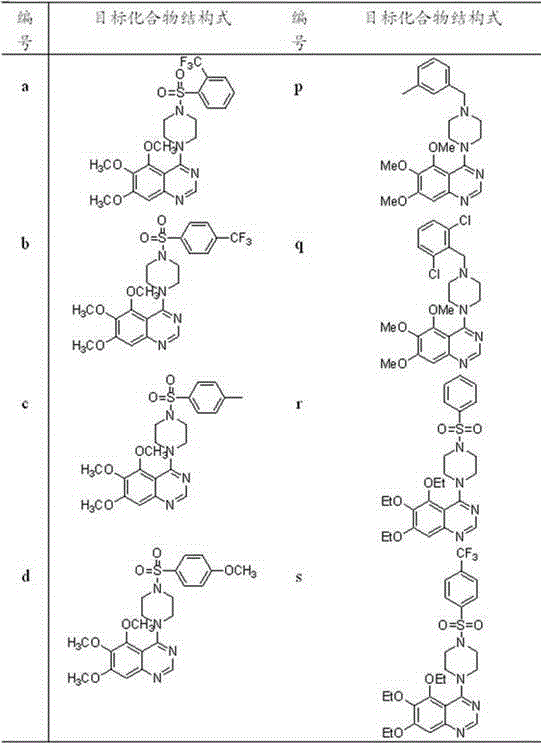
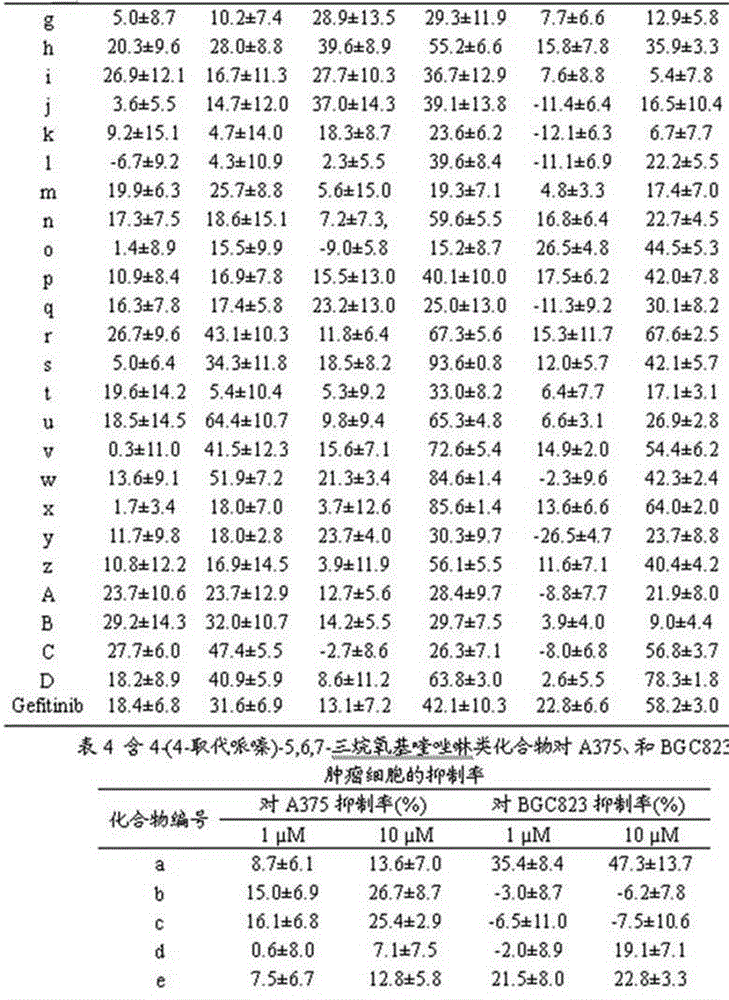
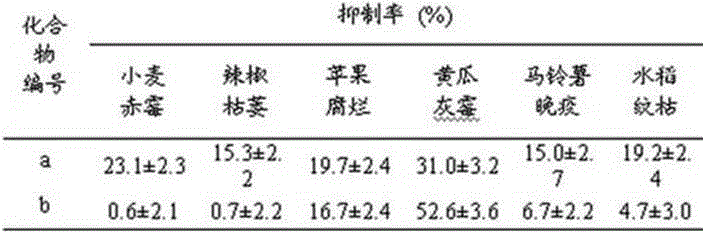
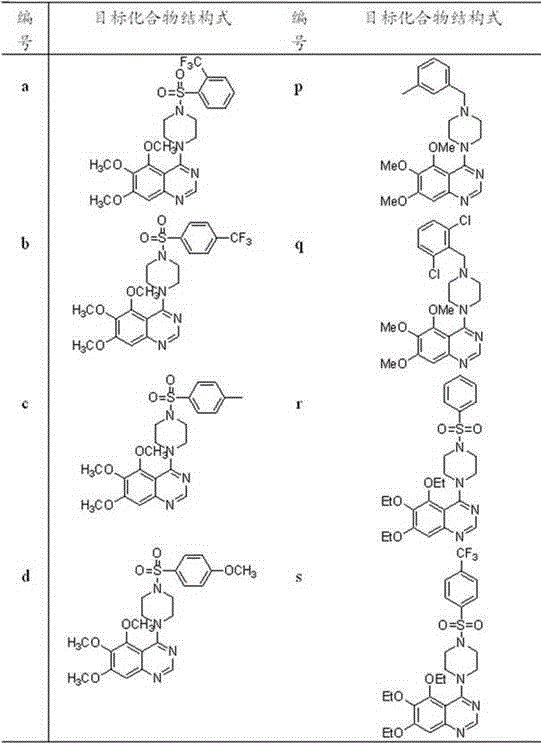
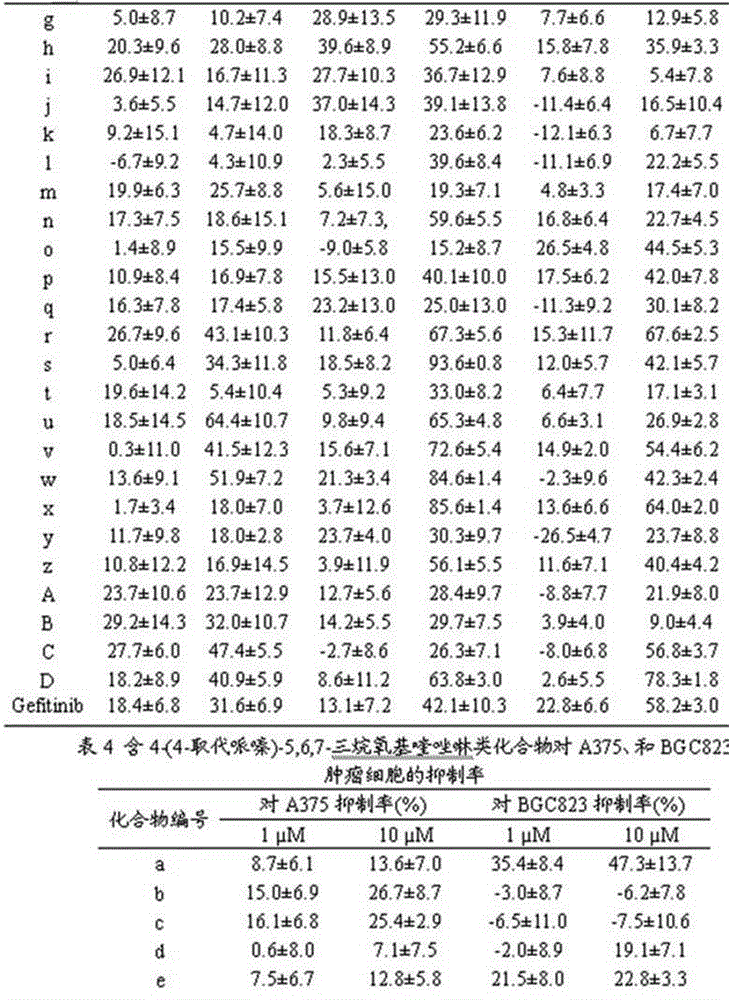
4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound as well as preparation method and application of 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound
The invention discloses a 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound as well as a preparation method and application of the 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound, wherein the compound structure is shown as the following general formula (I). A series of novel 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compounds are synthesized by using 2,3,4-trihydroxy benzoic acid, dimethyl sulfate, diethyl sulfate, methanol, sulfuric acid, nitric acid, hydrogen gas, formamide, phosphorus oxychloride, N-Boc piperazine, hydrochloric acid, aryl sulfonyl chloride and 4-aromatic (benzyl, pyridine and morpholine propyl) substituted piperazine as raw materials through multiple steps. The compound has a better anticancer effect and a plant fungus inhibition effect and can be used for preparing anticancer medicine and plant fungus resistance pesticide. (I) is shown as the accompanying drawing.
Owner:GUIZHOU UNIV
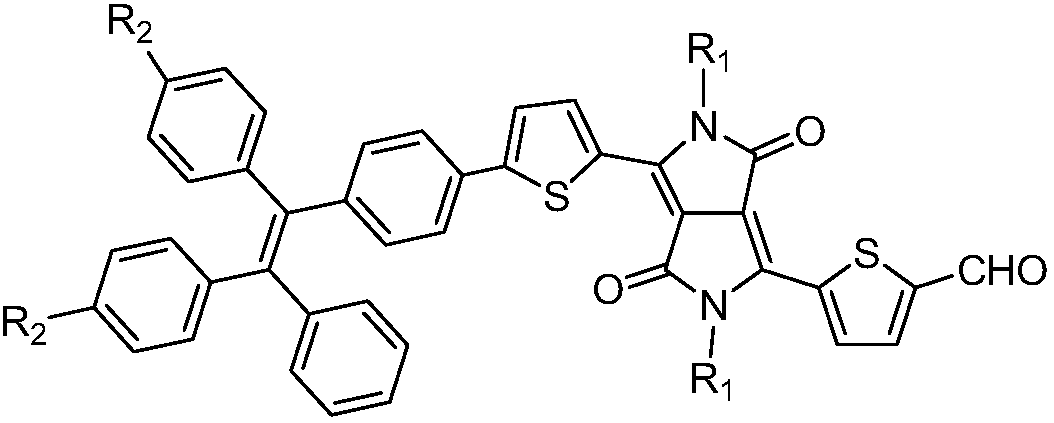
Aggregation-induced emission near-infrared emission diketopyrrolopyrrole compound and preparation method thereof
InactiveCN108912126AAdjustment rangeAdjust the fluorescence intensityOrganic chemistryLuminescent compositionsN dimethylformamideKetone
The invention discloses an aggregation-induced emission near-infrared emission diketopyrrolopyrrole compound and a preparation method thereof. The method comprises the steps that 3,6-dithiophene diketopyrrolopyrrole reacts with twice molar weight of alkyl bromide so as to obtain 2,5-dialkyl-3,6-dithienopyrrolopyrrole, then the 2,5-dialkyl-3,6-dithienopyrrolopyrrole sequentially undergoes Vilsmeierreaction with phosphorus oxychloride / N,N-dimethylformamide and undergoes substitution reaction with N-bromosuccinimide so as to obtain 2,5-dialkyl-3-(5-formyl)thienyl-6-(5-bromine)thienyl diketopyrrolopyrrole, and then the 2,5-dialkyl-3-(5-formyl)thienyl-6-(5-bromine)thienyl diketopyrrolopyrrole undergoes Suzuki reaction with tetraphenyl ethylene boric acid ester so as to obtain 2,5-dialkyl-3(4-((E)-2-phenyl-1,2-disubstituted styryl)phenyl)thienyl-6-(5-formyl)thienyl diketopyrrolopyrrole. The compound has the emission range greater than 650 nm and has the aggregation-induced emission property.
Owner:SOUTH CHINA UNIV OF TECH
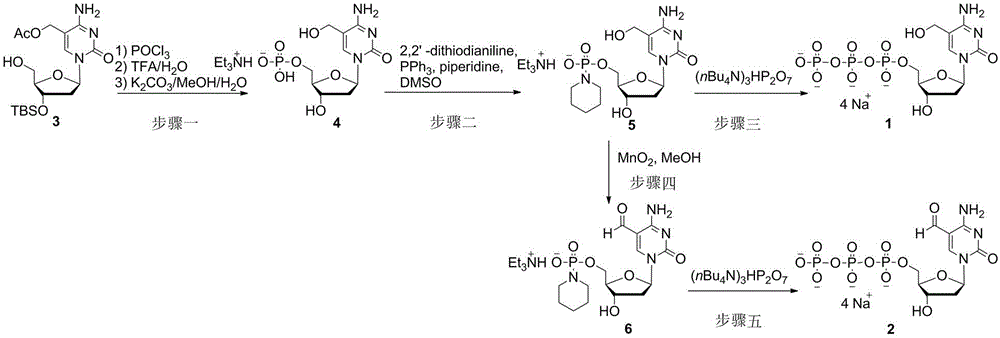
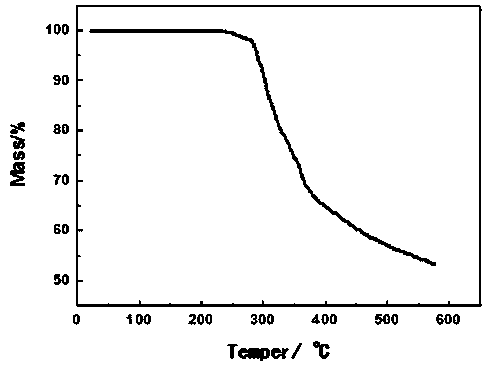
Method for synthesizing tetrasodium 5-hydroxymethyl and 5-aldehyde-2'-deoxycytidine triphosphate
InactiveCN104592334AHigh yieldEasy to operateSugar derivativesSugar derivatives preparationDeoxycytidine monophosphateChlorethoxyfos
The invention relates to a method for synthesizing tetrasodium 5-hydroxymethyl and 5-aldehyde-2'-deoxycytidine triphosphate. The method comprises the following steps of reacting 3'-tert-butyl-dimethylsilyl-5-acetoxymethyl-2'-deoxycytidine (3) and phosphorus oxychloride, removing TBS protecting groups with trifluoroacetic acid / water and removing acetyl groups with potassium carbonate / methanol / water to obtain triethylamine 5-hydroxymethyl-2'-deoxycytidine monophosphate (4); condensing the triethylamine 5-hydroxymethyl-2'-deoxycytidine monophosphate (4) and piperidine in the presence of 2,2'-dimercapto-diphenylamine / triphenylphosphine to obtain 5-hydroxymethyl-2'-deoxycytidine phosphoryl piperidine triethylamine salt (5); activating the 5-hydroxymethyl-2'-deoxycytidine phosphoryl piperidine triethylamine salt (5) and tris(tetrabutyl)ammonium pyrophosphate in the presence of 4,5-dicyanoimidazole to obtain tetrasodium 5-hydroxymethyl deoxycytidine triphosphate; and oxidizing the 5-hydroxymethyl-2'-deoxycytidine phosphoryl piperidine triethylamine salt (5) with activated manganese dioxide to obtain 5-aldehyde-2'-deoxycytidine phosphoryl piperidine triethylamine salt (6) and activating 5-aldehyde-2'-deoxycytidine phosphoryl piperidine triethylamine salt (6) and tris(tetrabutyl)ammonium pyrophosphate in the presence of 4,5-dicyanoimidazole to obtain tetrasodium 5-aldehyde-2'-deoxycytidine triphosphate.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Preparation method of halogen-free intrinsic flame-retardant waterborne polyurethane film
ActiveCN110483735AImprove flame retardant performanceImprove thermal stabilityGroup 5/15 element organic compoundsPolymer sciencePropanoic acid
The invention discloses a preparation method of halogen-free intrinsic flame-retardant waterborne polyurethane. The preparation method comprises the following steps: firstly, performing a reaction onphosphorus oxychloride with pentaerythritol to prepare an intermediate product with a diphosphoryl chloride functional group; performing a reaction on the intermediate product with diglycolamine and other substances, thereby preparing a micromolecular flame retardant containing phosphorus and nitrogen flame-retardant elements, and finally performing a reaction on the micromolecular flame retardantserving as a hard segment chain extender with polyether polyol, isophorone diisocyanate, 2,2-dimethylolpropionic acid and other substances to prepare the halogen-free intrinsic flame-retardant waterborne polyurethane. According to the invention, the reactive halogen-free intrinsic flame-retardant waterborne polyurethane is prepared by taking the halogen-free flame retardant as a reaction substance, so that the product is good in flame-retardant effect, simple in synthesis method and relatively low in cost, has very good practicability and economy, and has a good market prospect.
Owner:ZHONGYUAN ENGINEERING COLLEGE
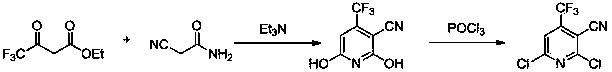
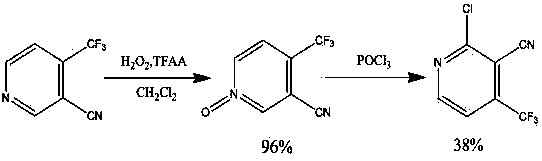
Preparation method for 2-chlorine-4-trifluoromethyl-3-cyanopyridine
ActiveCN103626697AHigh yieldSimple and fast operationOrganic chemistryChlorethoxyfosReaction intermediate
The invention discloses a preparation method for 2-chlorine-4-trifluoromethyl-3-cyanopyridine. The method comprises the following steps: 4, 6-dyhydroxy-4-trifluoromethyl-3-cyanopyridine is obtained through the condensation and amidation between ethyl trifluoro acetoacetate and cyanoacetamide, triethylamine is used as an alkali of the reaction, and ethyl alcohol, isopropanol and 1, 4-dioxane are used as reaction solvents; 2, 6-dichloro-4-trifluoromethyl-3-cyanopyridine is obtained in the way that the 4, 6-dyhydroxy-4-trifluoromethyl-3-cyanopyridine as a reaction intermediate is subjected to chlorination reaction, the reaction temperature is 50-100 DEG C, and phosphorus oxychloride is used as a solvent for the reaction; the 2-chlorine-4-trifluoromethyl-3-cyanopyridine is obtained in the way that the 2, 6-dichloro-4-trifluoromethyl-3-cyanopyridine (II) is subjected to reduction reaction under the catalysis of palladium carbon, the reaction conditions are normal temperature and normal pressure, the reaction solvent is methyl alcohol or ethyl alcohol. The preparation method is high in product yield, simple and convenient to operate, and low in reaction cost.
Owner:贵州威顿晶磷电子材料股份有限公司
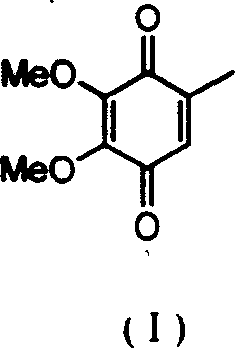
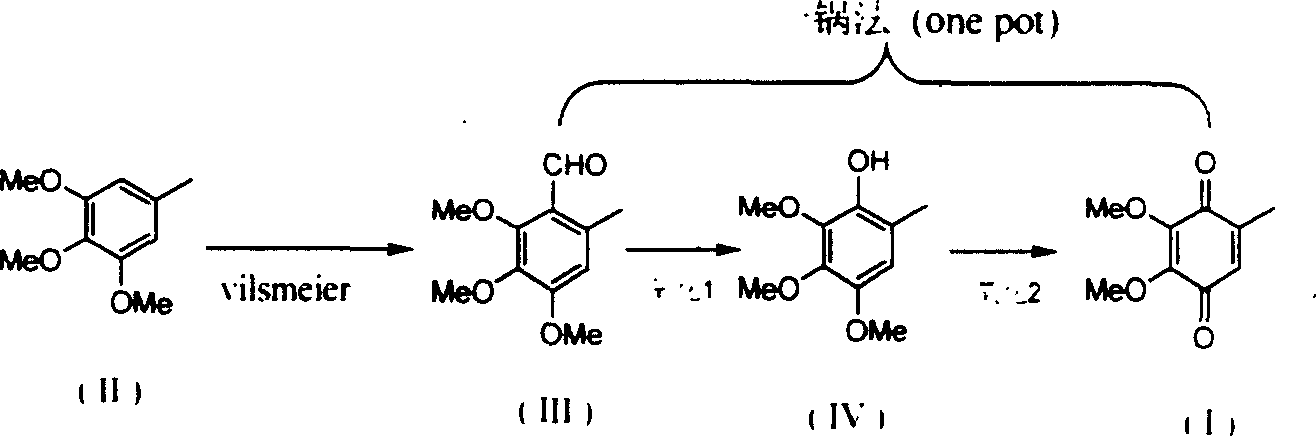
2,3-dimethoxy-5-methyl-1,4-benzoquinone ú¿ó±ú®preparation method
InactiveCN1544408ASolving Corrosion ProblemsEasy to recycleOrganic compound preparationQuinone preparationOrganic acidBenzaldehyde
The invention relates to a process for preparing 2,3-dimethoxy-5- methyl-1,4- benzophenone (I) by subjecting raw material 3,4,5-trimethoxytoluene (II) first with phosphorus oxytrichloride and N,N-dimethyl formamide to Vilsmeier reaction, obtaining 2,3,4-trimethoxyl-6- methyl- benzaldehyde (III) by introducing aldehydo onto phenyl ring, and oxidizing (III) into phenol (IV) with H2O2 at the presence of small amount organic acid catalysis at room temperate.
Owner:FUDAN UNIV
Synthetic method of artificial cytomembrane main ingredient 2-methacryloyloxy ethyl phosphorylcholine
ActiveCN107056834AThe synthetic route is simpleReduce stepsGroup 5/15 element organic compoundsPhosphatide foodstuff compositions2-methacryloyloxyethyl phosphorylcholinePhosphorylcholine
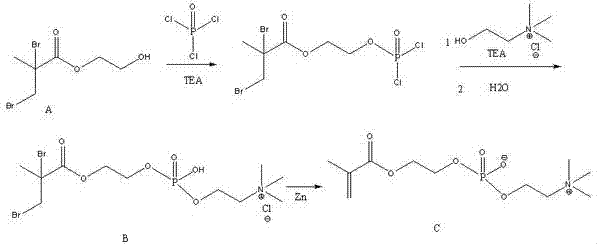
The invention provides a synthetic method of artificial cytomembrane main ingredient 2-methacryloyloxy ethyl phosphorylcholine. The synthetic method comprises the following steps: (1) dissolving phosphorus oxychloride in acetonitrile, adding triethylamine at 0 to 10 DEG C, dropwise adding 2,3-dibromo-2-hydroxyethyl methacrylate, reacting at -10 to 0 DEG C, adding triethylamine and choline chloride to facilitate the reaction at -10 to 0 DEG C, and obtaining bromophosphorylcholine after the reaction is ended; and (2) dissolving the bromophosphorylcholine obtained in the step (1) in ethanol, adding zinc powder, severely stirring, and obtaining 2-methacryloyloxyethyl phosphorylcholine. By adopting the synthetic method, a synthetic route of the original 2-methacryloyloxyethyl phosphorylcholine is simplified, no treatment is needed after the purification during the synthetic route, and the next operation can be directly carried out; severe conditions in the synthetic route are avoided, high-end equipment is not needed, uncontrollable factors during the synthetic route are reduced; raw materials are easy to obtain, the price is low, the cost is reduced, the use of solvents and raw materials with large toxicity is avoided, and the green synthesis is realized; and a foundation is set for the industrialization of the 2-methacryloyloxyethyl phosphorylcholine.
Owner:李铁龙
Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof
The invention relates to a tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof. The derivative is prepared by the steps that ethyl cyanoacetate serves as an initial raw material, a hydroxylamine compound (A) is generated under the action of sodium nitrite and phosphoric acid, ethyl 2-aminocyanoacetate (B) is obtained through reduction of sodium hydrosulfite and reacts with acetic anhydride and a Lawesson's reagent separately to obtain5-amino-4-formate thiazole compounds (D), NBS (N-bromosuccinimide) bromination is conducted, a 2-bromo-dihydropyrrolo[1,2-a]thiazolo[5,4-d]pyrimidinone compound (F) and a 2-bromo-7,8-dihydro-5H-pyridino[1,2-a]thiazolo[5,4-d]pyrimidin-10(6H)-one derivative (G) are obtained under the action of phosphorus oxychloride, and finally Suzuki coupling reaction is conducted to obtain 64 differently-substituted tricyclic thiazolo[5,4-d]pyrimidone derivatives H1-H32 and I1-I32. The inhibitory activity of the 64 compounds on acetylcholinesterase / butyrylcholinesterase in Alzheimer's disease and the antibacterial activity of Candida albicans, and the result shows that 46 compounds have strong inhibitory activity on acetylcholinesterase / butyrylcholinesterase, and 27 compounds have inhibitory activity on candida albicans.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
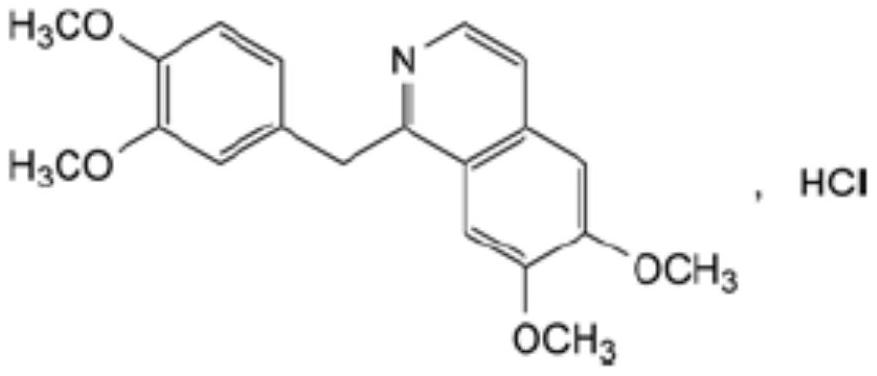
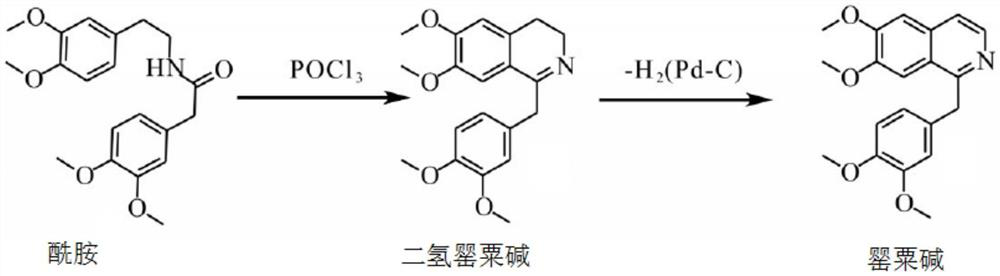
Preparation of powder injection pharmaceutical composition from high-purity papaverine hydrochloride
ActiveCN111848512AImprove pharmaceutical propertiesOrganic active ingredientsPowder deliveryPtru catalystChlorethoxyfos
The invention relates to preparation of a powder injection pharmaceutical composition from high-purity papaverine hydrochloride, in particular to a preparation method of papaverine hydrochloride. Themethod comprises the following steps of: heating 3, 4-dimethoxy-beta-phenyl-ethylamine and 3, 4-dimethoxy-phenyl-acetic acid to melting, and then carrying out reaction in a mixture of benzene and chlorethoxyfos to obtain 6, 7, 3', 4'-tetramethoxy-1-benzyl-dihydro-isoquinoline hydrochloride; then dissolving the wet product in tetrahydronaphthalene after the wet product becomes free alkali, and carrying out dehydrogenation reaction at 180DEG C in the presence of a Raney nickel catalyst; after dehydrogenation is finished, directly filtering the tetrahydronaphthalene reaction mixture from the Raney nickel catalyst into a mixture of a hydrochloric acid aqueous solution and methanol; filtering out precipitates, and performing recrystallizing from the ethanol-water solution in an inert gas environment to obtain off-white 6, 7, 3', 4'-tetramethoxy-1-benzyl isoquinoline hydrochloride, namely papaverine hydrochloride. The invention also relates to a papaverine hydrochloride powder injection pharmaceutical composition, and a preparation method and a quality detection method thereof. The invention achieves excellent technical effects as described in the specification.
Owner:山东北大高科华泰制药有限公司
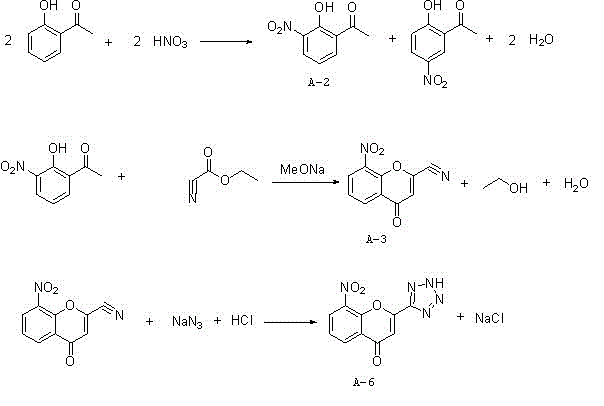
Synthesis method of 8-nitro-2-tetrazol-5-yl-4-oxo-4H-1-benzopyran
ActiveCN103980257ASimple operation processThe reaction steps are simpleOrganic chemistryChlorethoxyfosNitration
The invention relates to a synthesis method of 8-nitro-2-tetrazol-5-yl-4-oxo-4H-1-benzopyran. The method comprises the following steps: firstly nitrifying hydroxyacetophenone taken as a starting material with a nitric acid so as to generate a mixture of 2-hydroxy-3-nitroacetophenone and 2-hydroxy-5-nitroacetophenone; salifying the mixture by using an inorganic base, carrying out separation and purification and then hydrolyzing with an acid so as to obtain 2-hydroxy-3-nitroacetophenone (A-2); carrying out a cyclization reaction on the 2-hydroxy-3-nitroacetophenone (A-2) and ethyl cyanoformate so as to generate 8-nirto-2-cyano-4-oxo-4H-1-benzopyran (A-3); and finally, reacting with sodium azide so as to generate the 8-nitro-2-tetrazol-5-yl-4-oxo-4H-1-benzopyran (A-6). According to the method, phosphorus oxychloride with an application risk and large pollution and an ammonia gas with strong stimulating smell are not used, so that the production is safe and environment-friendly; and the generation of waste gases is reduced at the same time, so that the synthesis process is shortened. Thus, the operation is simplified; the production cost is lowered; and the 8-nitro-2-tetrazol-5-yl-4-oxo-4H-1-benzopyran is high in purity.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
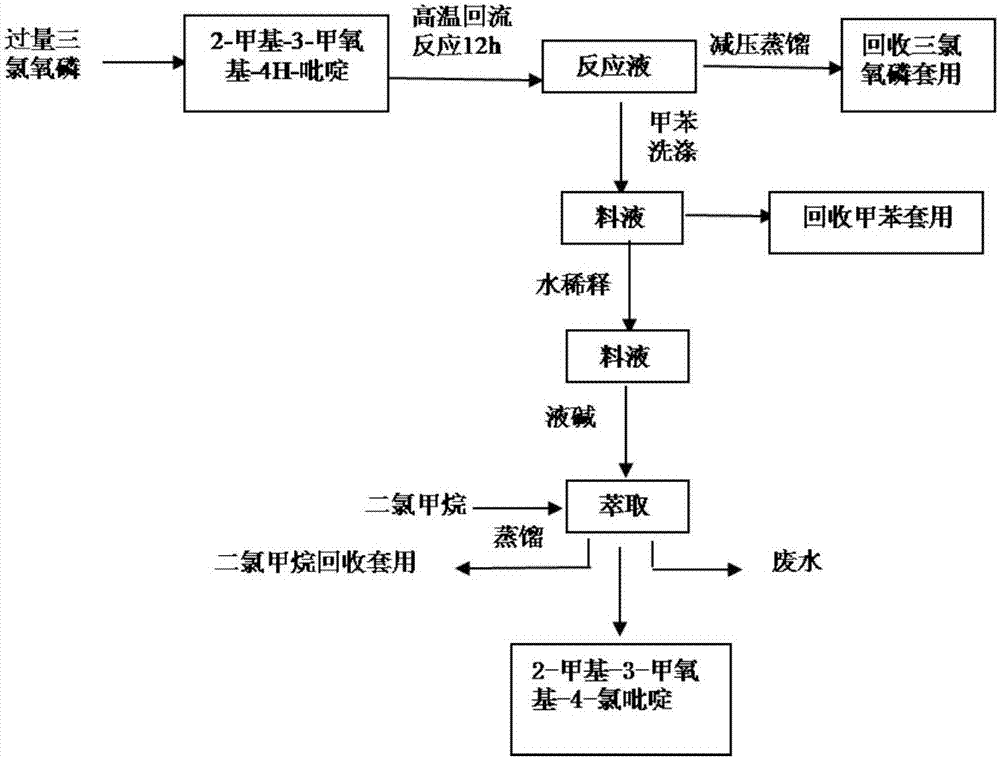
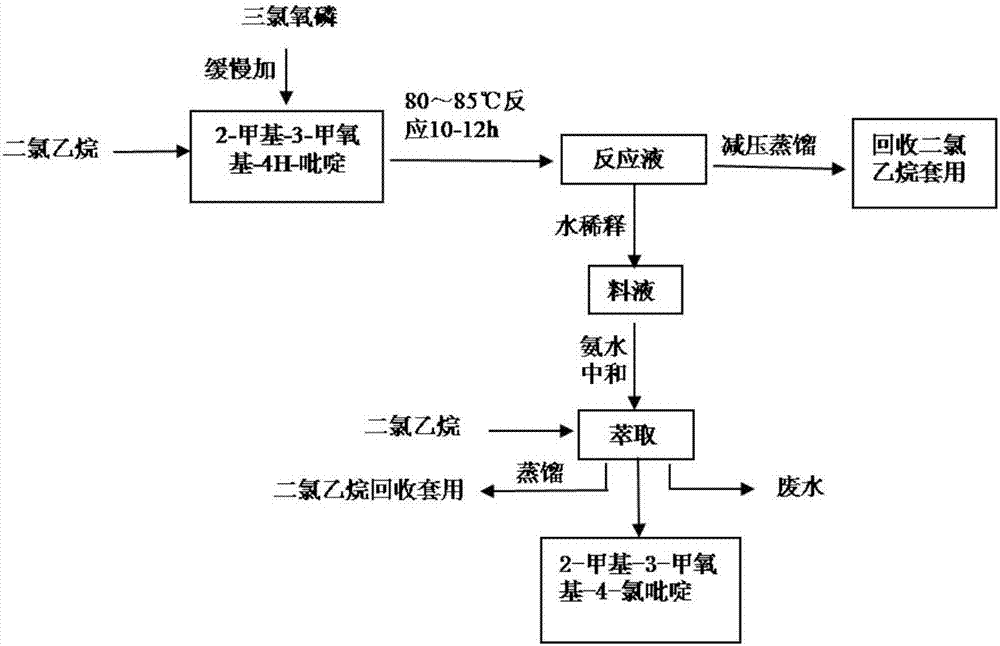
Preparation method of 2-methyl-3-methoxyl-4-chloropyridine
The invention discloses a preparation method of 2-methyl-3-methoxyl-4-chloropyridine. The preparation method comprises the following steps: adding 10 to 15 kg of 2-methyl-3-methoxyl-4H-pyridine into 50 to 75 kg of dichloroethane, and heating to dissolve the mixture; slowly adding 12 to 18 kg of phosphorus oxychloride into the solution under stirring; then performing heat preservation at 80 to 85 DEG C for 10 to 12 hours; performing reduced pressure distillation to recycle dichloroethane, cooling the material solution obtained by the reduced pressure distillation to 18 to 22 DEG C, and slowly adding the material solution into iced water for uniform stirring for hydrolysis; cooling the hydrolyzed material solution to 0 to 10 DEG C, adding an alkaline solution to adjust the pH of the materialsolution to 7 to 9, pouring the solution into a separating funnel for stewing for 1 to 2 hours, then collecting a lower-layer oily matter, extracting upper-layer liquid with an extractant, putting the extracted liquid into the collected oily matter, adding anhydrous sodium sulfate, performing drying and suction filtration, and performing reduced pressure evaporation to remove the extractant, thusobtaining the 2-methyl-3-methoxyl-4-chloropyridine. The preparation method is mild in reaction condition, convenient in technological operation, safe, reliable, high in product yield and low in cost.
Owner:CHUZHOU UNIV +1
Preparation method of environment-friendly 4-amino-2, 6-dimethoxy pyrimidine
ActiveCN114014815AFew reaction stepsEasy to operateOrganic chemistryChlorethoxyfosWater chlorination
The invention provides a preparation method of environment-friendly 4-amino-2, 6-dimethoxy pyrimidine, which comprises the following steps of: carrying out a cyclization reaction on 2-methoxy formamidine and methyl cyanoacetate as initial raw materials under a solvent-free condition to obtain 4-amino-2-methoxy-6-hydroxypyrimidine, carrying out a phosphorus oxychloride chlorination reaction to obtain 4-amino-2-methoxy-6-chloropyrimidine, and carrying out a methoxylation reaction to obtain a target product, namely, 4-amino-2, 6-dimethoxy pyrimidine. The reaction steps are few, the technological operation is simpler, the yield is guaranteed, and the method is suitable for industrial production. According to the preparation method disclosed by the invention, there is no need to use a large amount of solvent, reaction is performed under a solvent-free condition, and the method is high in safety and environment-friendly. Tthe usage amount of phosphorus oxychloride is small, and excessive phosphorus oxychloride is recycled, so that the cost of wastewater treatment is reduced, and the environmental pollution is small.
Owner:ZHEJIANG UNIVERSITY OF SCIENCE AND TECHNOLOGY
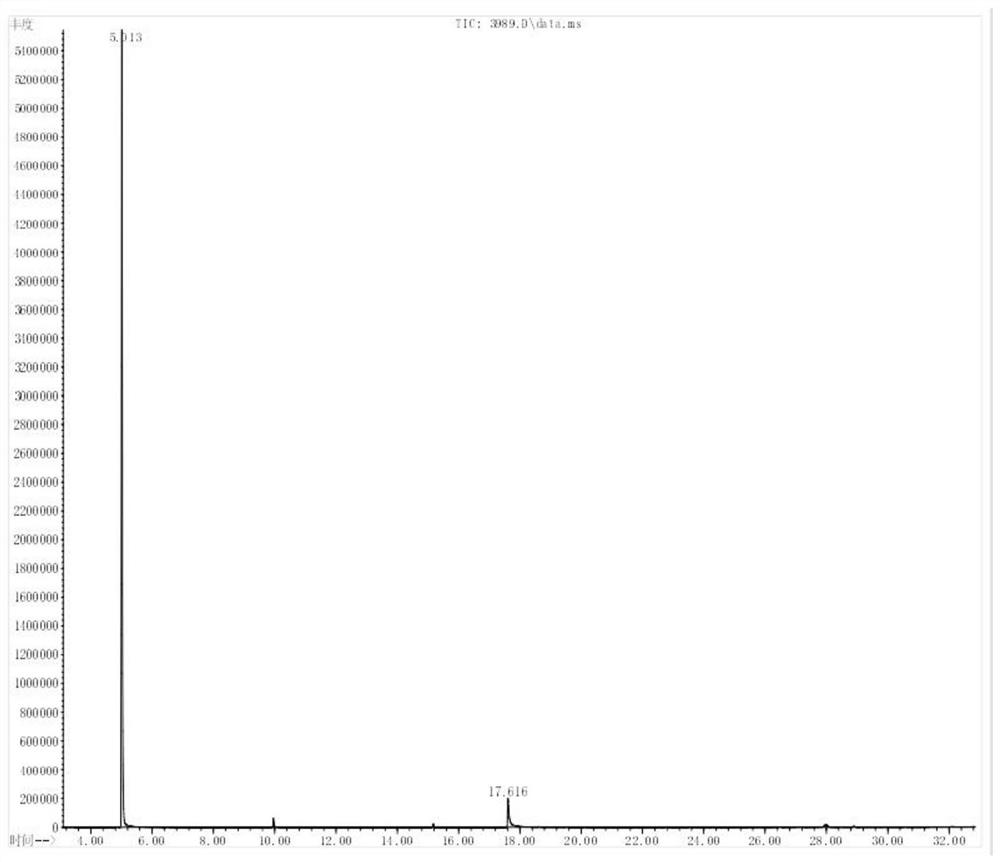
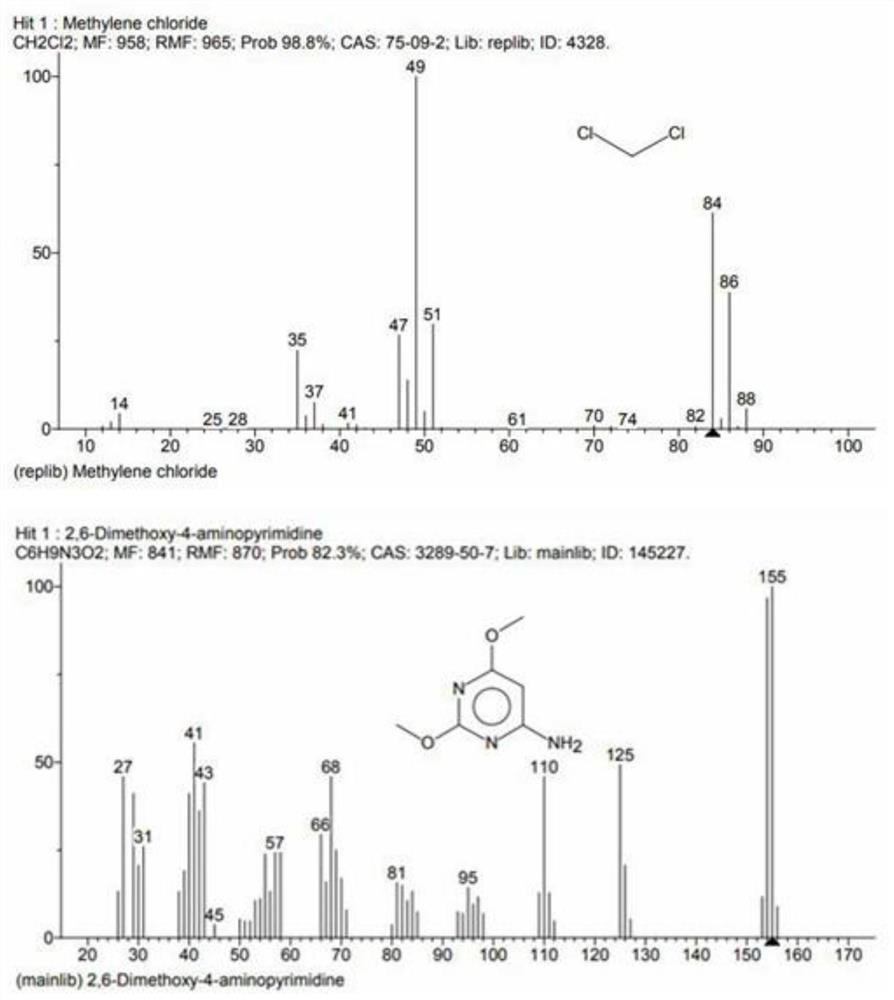
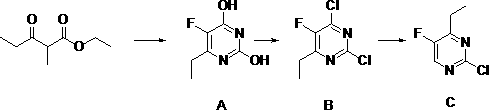
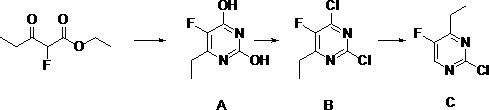
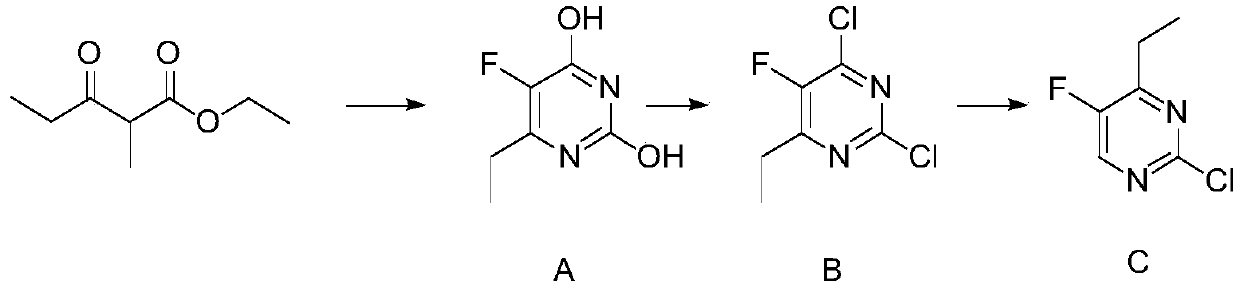
Synthetic method of 2-chlorine-5-fluorine-6-ethylpyrimidin-2-amine
The invention discloses a synthetic method of 2-chlorine-5-fluorine-6-ethylpyrimidin-2-amine. Step 1, sodium methoxide is dissolved in methyl alcohol, the temperature is lowered to the room temperature, carbamide is added, stirring is conducted after addition at the room temperature, 2-ethyl fluoropropioacetate is dropwise added, then the temperature is risen to reflux reaction for 2-3 hours, anda midbody A is obtained through purification; step 2, the midbody A, phosphorus oxychloride and organic alkali are mixed by weight ratio of 1 to 5-10 to 0.3-2, chlorination is conducted for 2-5 hoursat 25-100 DEG C, the mixture is cooled and subjected to vacuum concentration, extra phosphorus oxychloride is removed, after water is added for quenching, an organic solvent is used for extraction, drying and concentration are conducted, and then an intermediate product B is obtained; and step 3, ethyl alcohol, zinc powder and acetic acid are added in the intermediate product B, heating reflux reaction is conducted for 10-16 hours, the temperature is lowered, filtering is conducted, ethyl alcohol is removed by steaming, extraction is conducted through the organic solvent, a crude product is obtained through evaporation to dryness, and a pure product C is obtained through rectification under vacuum. According to the synthetic method of the 2-chlorine-5-fluorine-6-ethylpyrimidin-2-amine, theyield is increased, the reaction time is shortened, meanwhile the cost is lowered, and pollution on the environment is reduced.
Owner:南京普锐达医药科技有限公司
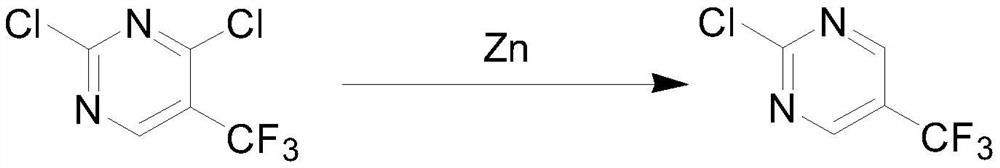
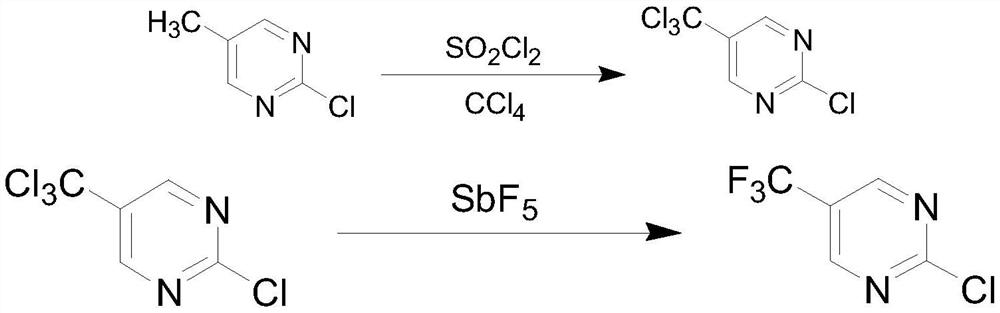
A kind of synthetic method of 2-fluoro-5-trifluoromethylpyrimidine
Owner:KINGCHEM LIAONING CHEMICAL CO LTD
A kind of synthetic method of cyclophosphamide
ActiveCN109535201BReduce usageSimple and fast operationGroup 5/15 element organic compoundsAcetic anhydrideChlorethoxyfos
The invention provides a synthetic method for cyclophosphoramide. According to the synthetic method, phosphorus oxychloride is slowly added into a mixed solution of dichloroethane, polyphosphoric acidand acetic anhydride, 3-amino propyl alcohol is dropwise added so as to prepare 2-chloro-2-oxo-[1.3.2] oxygen-nitrogen-phosphorus heterocyclic hexane; and dichloroethane and a 5a molecular sieve areadded, ammonia gas is introduced, heating is carried out at 4 atm to reach 120 DEG C, reaction is carried out for 2-2.5 hours, and treatment is carried out so as to obtain the cyclophosphoramide. Themethod has the advantages that the reaction conditions are relatively mild, and the yield and the content are high.
Owner:LIANYUNGANG GUIKE PHARMA
Preparation method of 2,4-dichloro-5-methoxypyrimidine
ActiveCN106187914AReduce dosageRaw materials are easy to getOrganic chemistryThiolMethyl methoxyacetate
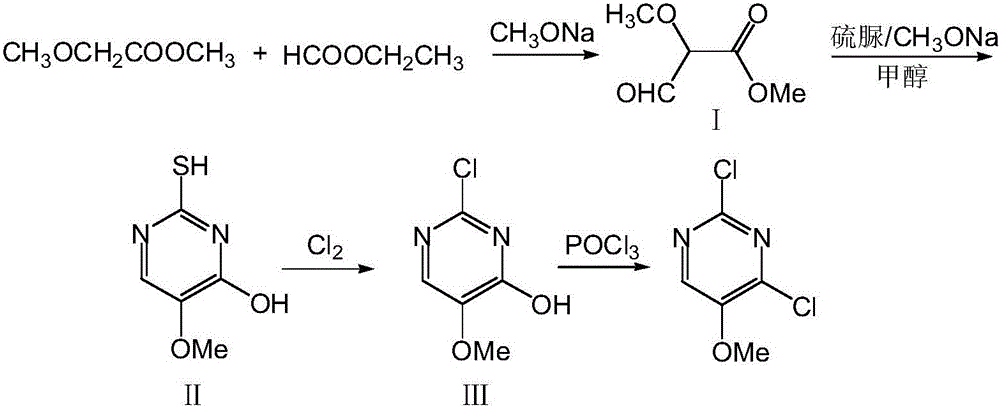
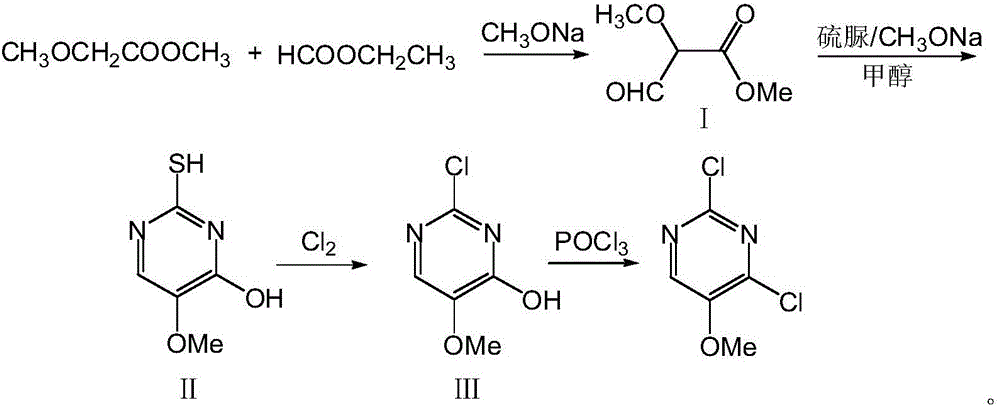
The invention relates to a preparation method of 2,4-dichloro-5-methoxypyrimidine, and belongs to the technical field of the preparation of intermediates of pesticides. According to the method, first, ethyl formate and methyl methoxyacetate are used as raw materials, and 2-thiol-4-hydroxyl-5-methoxypyrimidine is made through condensation and cyclization; afterwards, chlorination is carried out by using chlorine and phosphorus oxychloride successively, so as to obtain the 2,4-dichloro-5-methoxypyrimidine. All raw materials for the preparation method of the 2,4-dichloro-5-methoxypyrimidine are easily obtained; a synthetic route is novel; the yield of a ring formation step is high; the use level of a chlorinating agent is low; the preparation method of the 2,4-dichloro-5-methoxypyrimidine is suitable for industrialized production.
Owner:CHAMBROAD CHEM IND RES INST CO LTD
Preparation method of environment-friendly fomesafen
PendingCN113943233AImprove stabilityEasy to transportMolecular sieve catalystsSulfonic acid amide preparationBenzoic acidPtru catalyst
The invention discloses a preparation method of environment-friendly fomesafen, and relates to the field of synthesis of fomesafen. The fomesafen is synthesized from 5-[2-chloro-4-(trifluoromethyl) phenoxy]-2-nitrobenzoic acid, methanesulfonamide and triphosgene under the action of dichloroethane. According to the invention, by changing an acylating agent and a synthesis process, the problem of environmental pollution caused by taking phosphorus oxychloride as the acylating agent in the prior art is solved, and safe and environment-friendly synthesis and preparation of fomesafen are realized; and by improving the catalyst, the conversion rate and the reaction speed are improved, and the influence of the color of the chlorinated metal salt on the product quality is reduced.
Owner:山东科源化工有限公司
High-flame-retardant artistic coating and preparation method thereof
ActiveCN112251100AImprove flame retardant performanceEasy constructionFireproof paintsMulticolor effect coatingsPolymer scienceMeth-
The invention discloses a high-flame-retardant artistic coating which is composed of the following raw materials in parts by weight: 10-50 parts of acrylic copolymer; 0.1-5 parts of a rheological additive; 0.1-0.6 part of a dispersing agent; 0.1-0.6 part of a wetting agent; 2-10 parts of a coalescing agent; 1-5 parts of an antifreeze agent; 0.1-0.5 part of a defoaming agent; 0.1-0.3 part of a preservative; 0.1-0.5 part of a mildew preventive; 0.1-0.8 of a pH regulator; 1-40 parts of water; 10-30 parts of a filler; 10-20 parts of a pearlescent effect material. The dispersing agent is flame-retardant polyether polyol and is prepared from 100-150 parts of halogen-containing epoxy compound monomer, 2-5 parts of initiator and 5-20 parts of flame-retardant catalyst, wherein the halogen-containing epoxy compound monomer is selected from one or more of 4, 4, 4-trichloro-1, 2-epoxybutane, 4, 4, 4, -tribromo-1, 2-epoxybutane or epoxy chloropropane; the flame-retardant catalyst is selected from one or more of phosphorus oxychloride, antimony pentoxide and tetrakis (hydroxymethyl) phosphonium chloride; and the initiator is selected from propylene glycol. The coating has the advantages of excellent scratch resistance, high hardness, good hand feeling and strong layering sense.
Owner:GUANGDONG MAYDOS BUILDING MATERIALS LTD CO
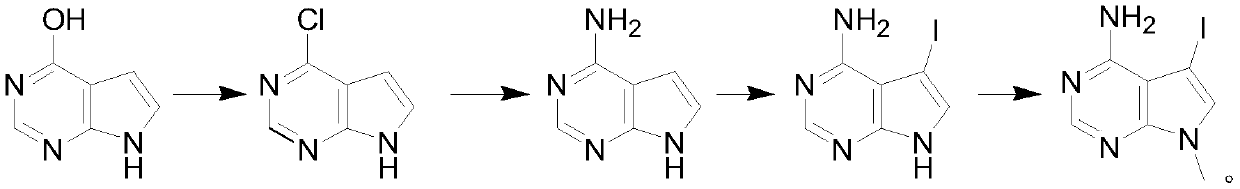
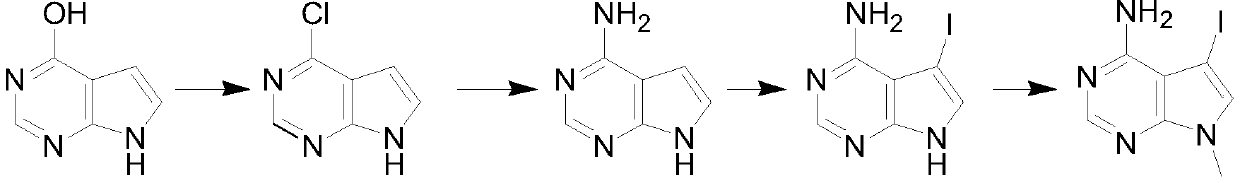
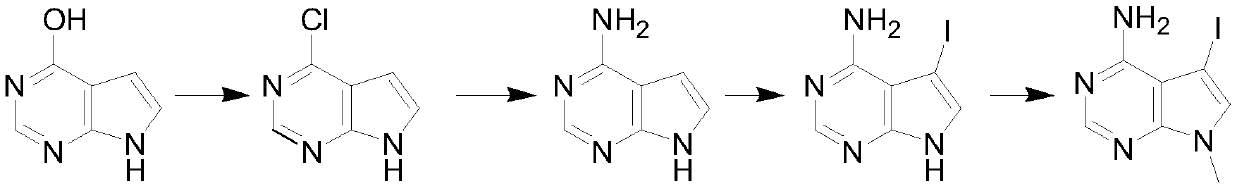
Synthesis method of 4-chloropyrrolopyrimidine compound
The invention particularly relates to a synthetic method of a 4-chloropyrrolopyrimidine compound. The method comprises the following steps: step 1, mixing 4-hydroxypyrrolopyrimidine, phosphorus oxychloride and an organic alkali according to a weight ratio of 1: (5-10): (3-7), carrying out a chlorination reaction in a certain temperature range, carrying out reduced pressure concentration to removeredundant phosphorus oxychloride, quenching, extracting, drying, and concentrating to obtain 4-chloropyrrolopyrimidine; step 2, dissolving 4-chloropyrrolopyrimidine in ethanol, starting to dropwise add an ethanol solution of ammonia, carrying out a reaction at a constant temperature, and removing excess ethanol to obtain 4-aminopyrrolopyrimidine; step 3, dissolving 4-aminopyrrolopyrimidine in dichloromethane, adding iodo-succinimide in batches at the room temperature, filtering to remove solid insoluble substances, and completely evaporating the filtrate to obtain 4-amino-5-iodopyrrolopyrimidine; and step 4, dissolving 4-amino-5-iodopyrrolopyrimidine in DMF, adding cesium carbonate and methyl iodide, carrying out a reflux reaction for 12 h, cooling, filtering, and evaporating to obtain theproduct namely 4-amino-5-iodine-7-methylpyrrolopyrimidine.
Owner:南京普锐达医药科技有限公司
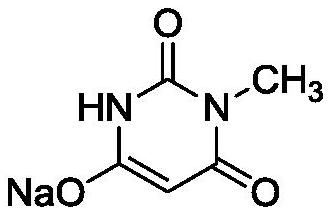
The preparation method of 6-chloro-3-methyluracil
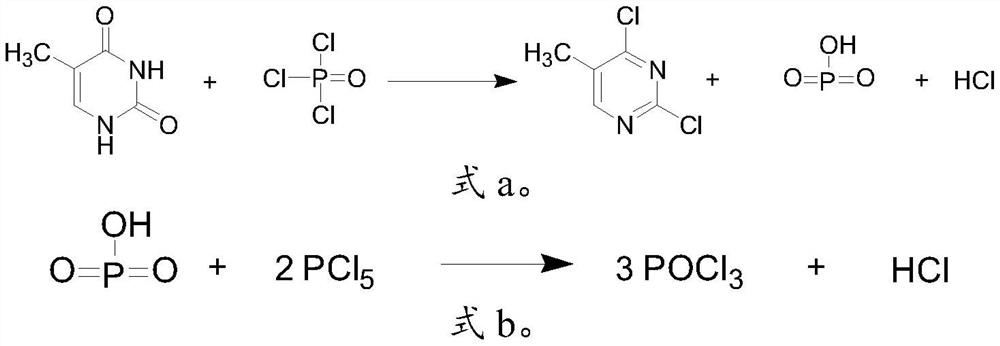
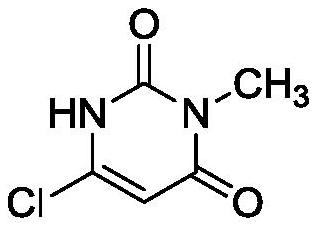
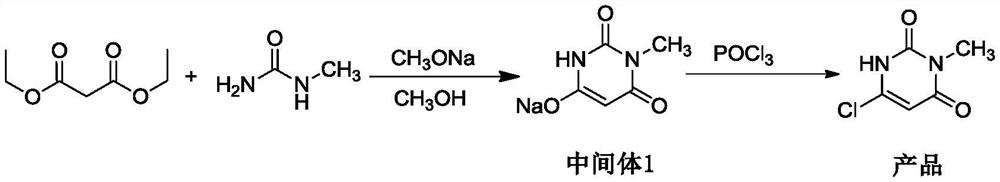
The invention belongs to the technical field of medicine chemosynthesis, and relates to a preparation method of 6-chloro-3-methyluracil. The method comprises the following steps that 1, methylurea, anorganic solvent and alkali are added into a reaction container for stirring and dissolution, then malonic acid or malonic ester is added, heating and reflux are conducted, then cooling is conducted,acid is added to adjust a pH value of reaction liquid, water is added, the reaction liquid is cooled, suction filtration is conducted, and drying is conducted to obtain a white to off-white first intermittent in a shape of loose powder; 2, phosphorus oxychloride is used for chloridizing the first intermittent to obtain a crude product; 3, the crude product prepared in the step 2 is subjected to decoloration by means of activated carbon to obtain a finished product. According to the preparation method of the 6-chloro-3-methyluracil, the product in the step 1 precipitates in a form of sodium salt, that is to say, the first intermittent is 1-sodium methylbarbiturate, compared with the prior art where a first intermittent precipitates in a form of 1-methylbarbituric acid, the preparation method of the 6-chloro-3-methyluracil has the advantages that the yield is obviously improved, moreover, in the step 2, acetonitrile is used as the solvent, the phosphorus oxychloride usage is reduced, post-treatment is convenient, and meanwhile, the product has a good crystal form, and is easy to filter.
Owner:ZHEJIANG XIANFENG TECH
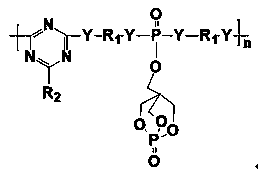
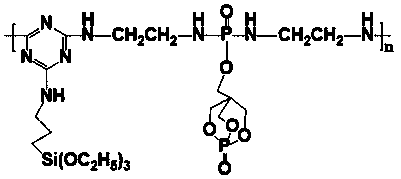
Phosphorus-nitrogen-silicon intumescent flame retardant containing triazine ring and cage structure and its synthesis method
The invention relates to a phosphorus-nitrogen-silicon intumescent flame retardant containing a triazine ring and a cage structure and a synthesis method of the intumescent flame retardant. The synthesis method comprises the steps as follows: a solvent is added to a container, phosphorus oxychloride is added and stirred to be dispersed, PEPA (1-oxy phospha-4-(hydroxymethyl)-2,6,7-trioxabicyclo[2.2.2]octane) and an acid-binding agent are dropwise added step by step, and a PEPA-containing unitary substituent is obtained; substances with diamine or dihydric alcohol are added to the other container and mixed with the solvent and the acid-binding agent, the PEPA-containing unitary substituent is slowly dropwise added, and a phosphorous-containing diamine intermediate is obtained after separation; the solvent is added to a container provided with a reflux condenser and the like, cyanuric chloride is added and stirred to be dispersed, an amino silane coupling agent and the acid-binding agent are dropwise added step by step, and a silicon-containing unitary substituent is obtained; the mixture of the phosphorous-containing diamine intermediate and the acid-binding agent is slowly dropwise added, cooling, washing and drying are performed, and a powdery solid, namely, the phosphorus-nitrogen-silicon intumescent flame retardant containing the triazine ring and the cage structure, is obtained. The phosphorus-nitrogen-silicon intumescent flame retardant has the advantages that the flame retardant has certain polymerization degree, high molecular weight, higher charcoal forming amount and better thermal stability, the proportion of acid sources, carbon sources and gas sources in molecular structures is relatively proper and the like.
Owner:SHUNDE POLYTECHNIC
4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound as well as preparation method and application of 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound
The invention discloses a 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound as well as a preparation method and application of the 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound, wherein the compound structure is shown as the following general formula (I). A series of novel 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compounds are synthesized by using 2,3,4-trihydroxy benzoic acid, dimethyl sulfate, diethyl sulfate, methanol, sulfuric acid, nitric acid, hydrogen gas, formamide, phosphorus oxychloride, N-Boc piperazine, hydrochloric acid, aryl sulfonyl chloride and 4-aromatic (benzyl, pyridine and morpholine propyl) substituted piperazine as raw materials through multiple steps. The compound has a better anticancer effect and a plant fungus inhibition effect and can be used for preparing anticancer medicine and plant fungus resistance pesticide. (I) is shown as the accompanying drawing.
Owner:GUIZHOU UNIV
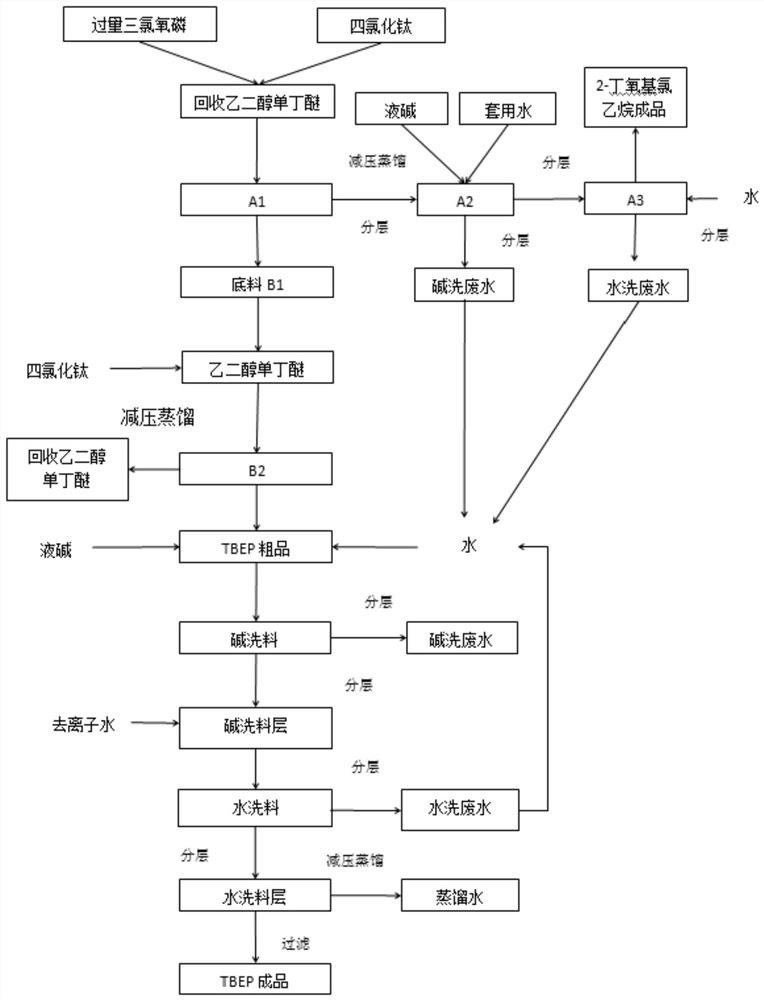
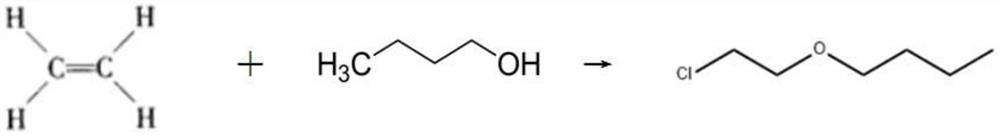
Separation and purification process of by-product 2-butoxyethyl chloride in production process of tris (butoxyethyl) phosphate
PendingCN114853580AHigh purityLow input costEther separation/purificationPhosphorus organic compoundsTitanium chlorideEthyleneglycol monobutyl ether
The invention discloses a separation and purification process of a byproduct 2-butoxyethyl chloride in the production process of tris (butoxyethyl) phosphate, which comprises the following steps of: adding titanium tetrachloride into recycled ethylene glycol monobutyl ether containing 2-butoxyethyl chloride with the concentration of more than or equal to 30%, completely dissolving, cooling, dropwise adding excessive phosphorus oxychloride, and reacting at the temperature of between 20 and 30 DEG C to obtain the 2-butoxyethyl phosphate. After dropwise adding is completed, heat preservation is conducted for a period of time, heating continues, ethylene glycol monobutyl ether in the materials is thoroughly reacted to obtain butoxyphosphoric acid diacyl chloride, reduced pressure distillation is conducted, a 2-butoxychloroethane crude product and a base material are separated out, the base material is used for preparing TBEP, liquid caustic soda and water are added into the 2-butoxychloroethane crude product, stirring alkali washing is conducted, and the 2-butoxychloroethane crude product is obtained. And standing to separate out a discharge layer and an alkali washing wastewater layer, adding water into the separated discharge layer, stirring and washing, standing, and separating out a 2-butoxychloroethane finished product and washing wastewater. The method disclosed by the invention is tightly combined with a tris (butoxyethyl) phosphate production process, and ethylene glycol monobutyl ether is intermittently and intensively treated and recycled by using the existing equipment.
Owner:ZHEJIANG WANSHENG
Preparation method of mepanipyrim
InactiveCN104003944AThe reaction process is simpleMild reaction conditionsOrganic chemistryChemical synthesisChlorethoxyfos
The invention provides a preparation method of mepanipyrim and belongs to the field of pesticide chemical synthesis technologies. The preparation method of the mepanipyrim comprises the following steps: firstly, reacting phenyl guanidine salt with ethyl acetoacetate so as to prepare phenylamino pyrimidone, reacting the phenylamino pyrimidone with phosphorus oxychloride so as to prepare 2-chloro-pyrimidine phenylamine; subsequently, carrying out coupling and crossing reaction between the 2-chloro-pyrimidine phenylamine and alkyne so as to prepare mepanipyrim. The preparation method of mepanipyrim has the advantages that the used raw materials and reagents are cheap and easily available; the reaction process is simple, the reaction conditions are mild, the cost is low, the yield is high, a good condition is created for industrial large-scale production and commercialization of the products.
Owner:NORTHWEST NORMAL UNIVERSITY
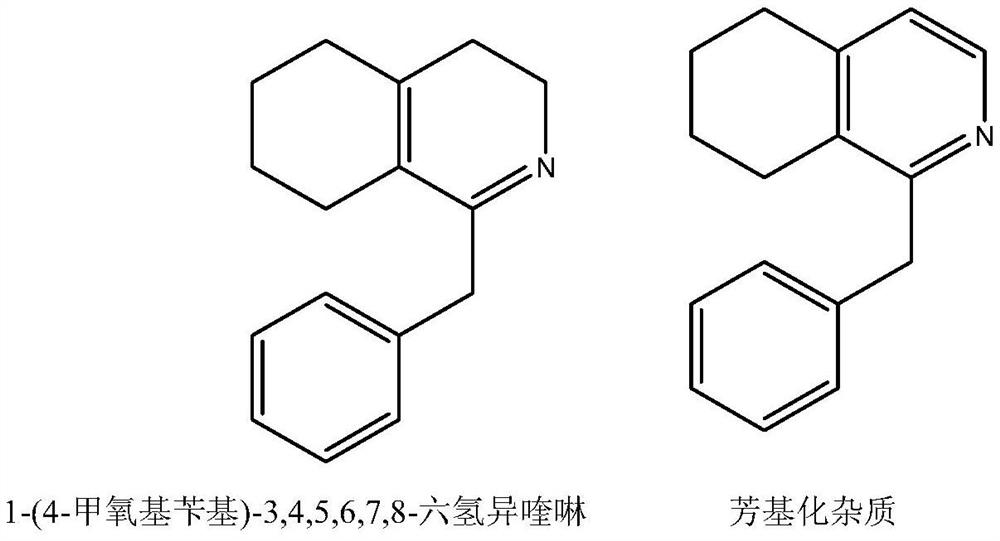
The purification method of 1-(4-methoxybenzyl)-3,4,5,6,7,8-hexahydroisoquinoline salt
Owner:启东东岳药业有限公司
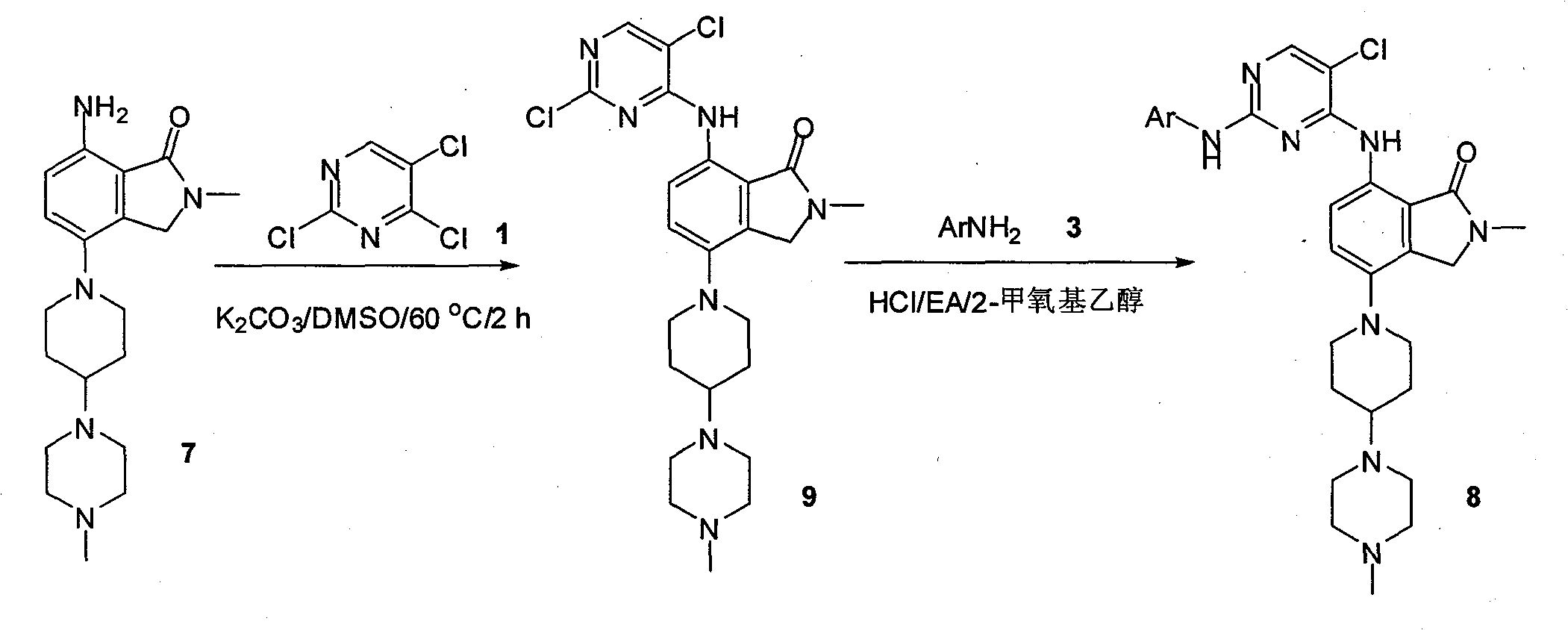
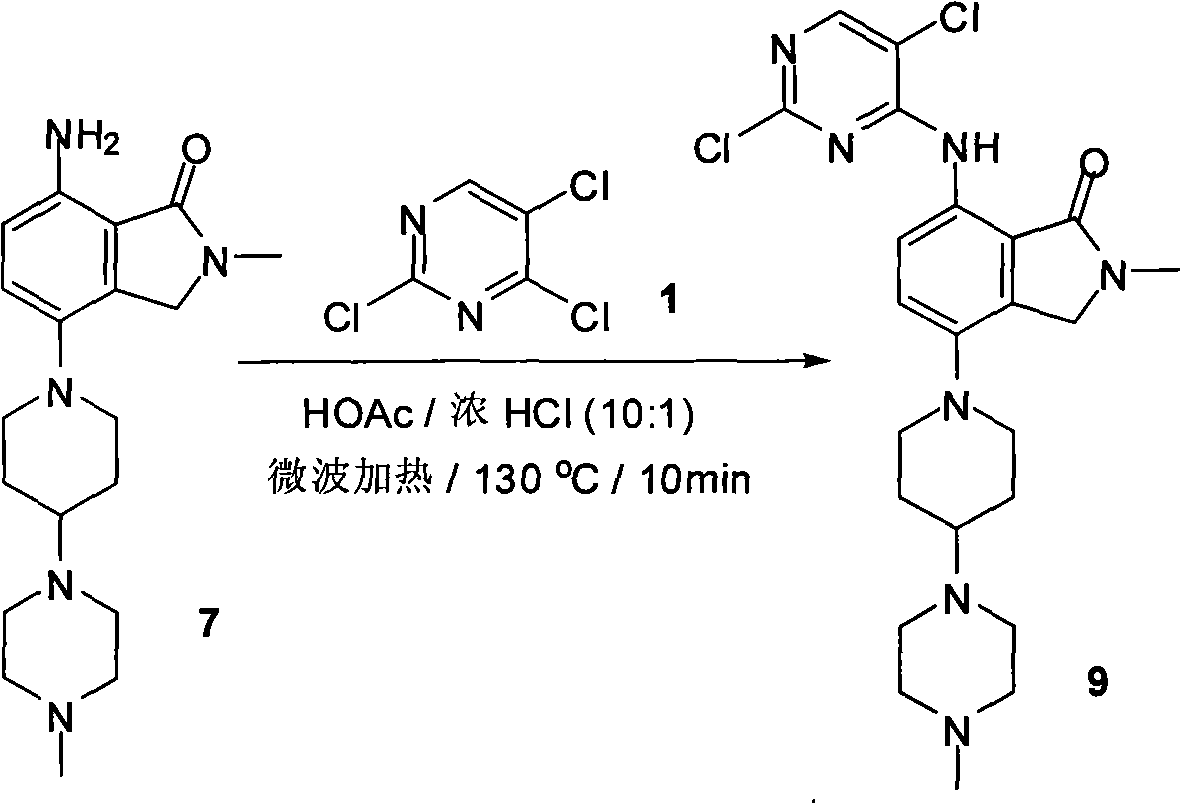
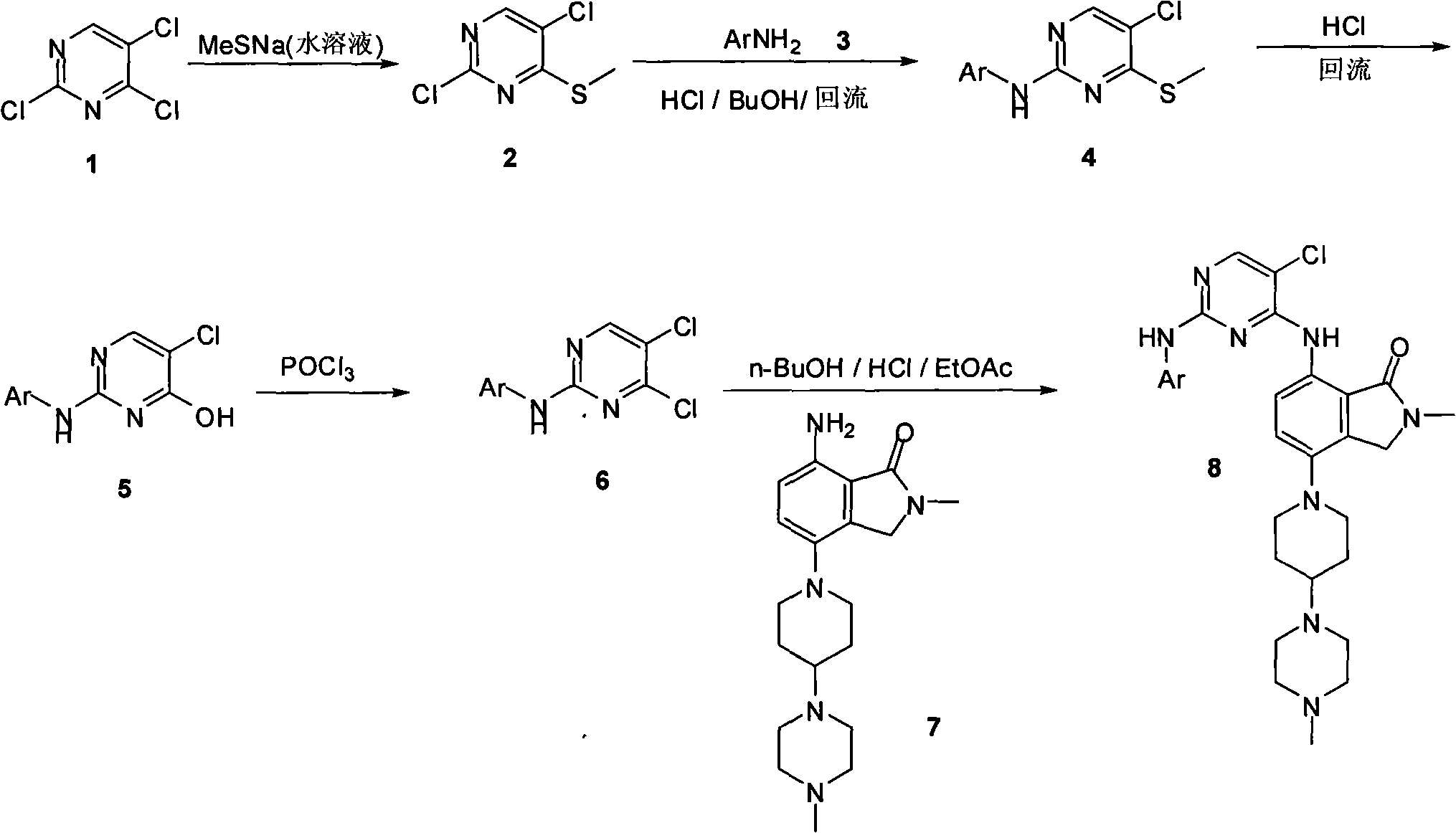
Synthesizing method of 2-methyl-7-(substituted pyrimidine-4-amino)-4-(substituted piperazine-1-base) piperidine-1-base) isoindoline-1-ketone and intermediate thereof
The invention relates to an synthesizing method of 2-methyl-7-( substituted pyrimidine-4-amino)-4-(substituted piperazine-1-base) piperidine-1-base) isoindoline-1-ketone and an intermediate thereof, mainly solving the technical problems of overlength single-line synthetic route, difficult separation and purification, low productivity, high synthesizing cost, narrow applicability, and the like of the traditional synthesizing method. The process method comprises the following steps of: carrying out substitution reaction on 2,4,5-trichloropyrimidine as a raw material and sodium methyl mercaptideto generate the intermediate, i.e. 2,5-dichloro-4-methylthiopyrimidine; then carrying out the substitution reaction with aromatic amine to generate the intermediate, i.e. 2-aromatic amino-4-methylthio-5-chloropyrimidine; then hydrolyzing the methylthio to generate the intermediate, i.e. 2-aromatic amino-4-hydroxy-5-chloropyrimidine; enabling a hydroxyl compound to generate dichloride under the action of phosphorus oxychloride; and finally carrying out the substitution reaction with amine to obtain the 2-methyl-7-(5-chlorine-2-aromatic amido-pyrimidine-4-amino)-4-(4-(4-methyl piperazine-1-base) piperidine-1-base) isoindoline-1-ketone under the action of hydrogen chloride ethyl acetate.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
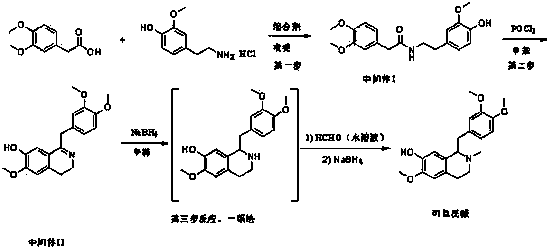
Synthetic method for codamine
InactiveCN103965109AEasy to operateExperiment operation is simpleOrganic chemistryChemical synthesisChlorethoxyfos
The invention provides a chemical synthetic method for codamine. According to the method, 3,4-dimethoxy phenylacetic acid and 4-hydroxy-3-methoxy phenylethylamine hydrochloride are used as starting raw materials, three steps of reactions consisting of acid amide condensation for preparation of an intermediate I, dehydration with phosphorous oxychloride for preparation of an intermediate II and reduction of the intermediate II are carried out to prepare codamine, and overall yield is 34%. The method provided by the invention has the characteristics of simple operation, easy availability of raw materials, short reaction time, etc.
Owner:公安部禁毒情报技术中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine](https://images-eureka.patsnap.com/patent_img/f9d04bb3-ee16-4c50-a3dd-18ac6fa044c3/BDA00003068755500031.PNG)
![Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine](https://images-eureka.patsnap.com/patent_img/f9d04bb3-ee16-4c50-a3dd-18ac6fa044c3/BDA00003068755500071.PNG)
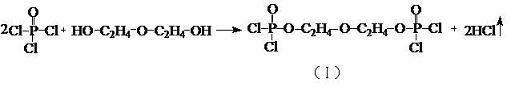
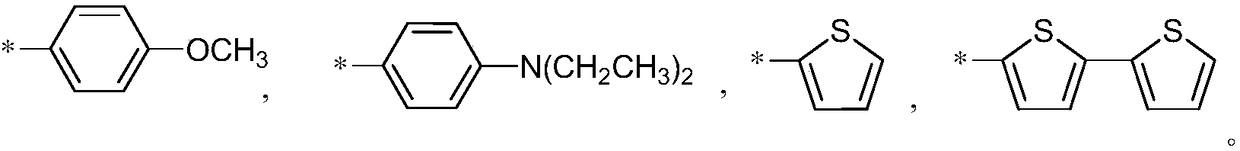
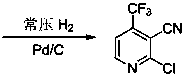
![Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof](https://images-eureka.patsnap.com/patent_img/2b2a1369-2132-497a-9110-e6d46991144f/FDA0003323019360000011.png)
![Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof](https://images-eureka.patsnap.com/patent_img/2b2a1369-2132-497a-9110-e6d46991144f/FDA0003323019360000021.png)
![Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof](https://images-eureka.patsnap.com/patent_img/2b2a1369-2132-497a-9110-e6d46991144f/BDA0003323019370000041.png)