Method for synthesizing tetrasodium 5-hydroxymethyl and 5-aldehyde-2'-deoxycytidine triphosphate
A technology of hydroxymethyl deoxycytidine triphosphate tetrasodium salt and aldehyde deoxycytidine triphosphate tetrasodium salt, applied in 5-hydroxymethyl and 5-formyl-2'-deoxycytidine triphosphate tetrasodium salt In the field of salt synthesis, it can solve the problems of unavailable 5-methyl-2'-deoxycytidine triphosphate raw materials and difficult control conditions, and achieve high practical application value, easy method, simple and easy-to-obtain raw materials and reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
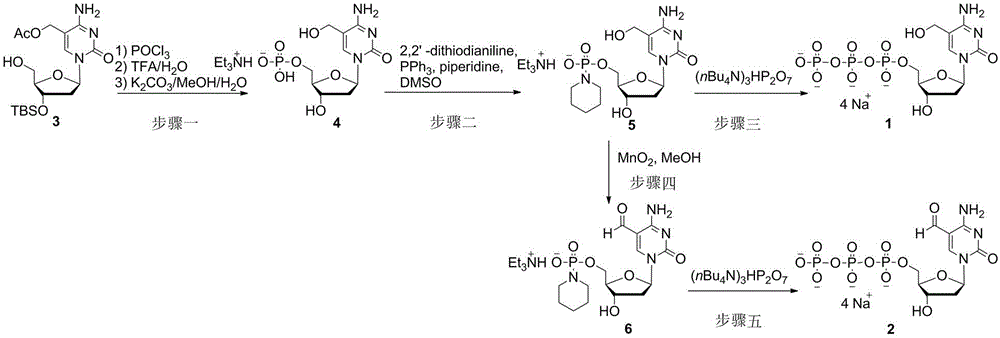
[0008] Example 1: 5-Hydroxymethyl-2′-deoxycytidine 5′-triphosphate tetrasodium salt ( 1 )Synthesis
[0009] 1) 5-Hydroxymethyl-2′-deoxycytidine 5′-triethylamine monophosphate ( 4 ) synthesis: under argon protection, the 3 (515 mg, 1.25 mmol) was dissolved in dry trimethyl phosphate (10 mL), and phosphorus trichloride (380 mg, 2.5 mmol) was added to react at 0 °C for 3 hours. Slowly added 0.1 M triethylamine carbonate buffer solution (40 mL) to quench the reaction, then extracted three times with diethyl ether (40 mL), and concentrated the aqueous phase under reduced pressure. Add trifluoroacetic acid (5 mL) and water (5 mL) to the residual oil, react at 20 °C for 1 hour, and concentrate the reaction solution under reduced pressure. Afterwards, methanol (10 mL), potassium carbonate (345 mg, 2.5 mmol) and water (1 mL) were added to the concentrate, reacted at 20 °C for 1 hour, and the reaction solution was concentrated under reduced pressure to obtain a crude product. Usin...
Embodiment 2
[0012] Example 2: 5-Formyl-2′-deoxycytidine 5′-triphosphate tetrasodium salt ( 2 )Synthesis
[0013] 1) 5-formyl-2′-deoxycytidine 5′-phosphoryl piperidine triethylamine salt ( 6 ) synthesis: the 5 (101 mg, 0.2 mmol) was dissolved in methanol (5 mL), activated manganese dioxide (174 mg, 2 mmol) was added, heated to 50 °C for 24 hours, the manganese dioxide was removed by filtration, and the filtrate was concentrated under reduced pressure. Column chromatography separation (dichloromethane: methanol = 5: 1, containing 0.5% triethylamine) to obtain a white solid ( 6 ) 85 mg, yield 84%.
[0014] 2) 5-formyl-2′-deoxycytidine 5′-triphosphate tetrasodium salt ( 2 ) synthesis: under argon protection, the 6 (50 mg, 0.1 mmol) dissolved in dry N , N -Dimethylformamide (2 mL), add tri(tetrabutyl)ammonium pyrophosphate (180 mg, 0.2 mmol) and 4,5-dicyanoimidazole (71 mg, 0.6 mmol) at 20 °C . After reacting for 6 hours, the solvent was removed under reduced pressure, 3 M sodium ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com