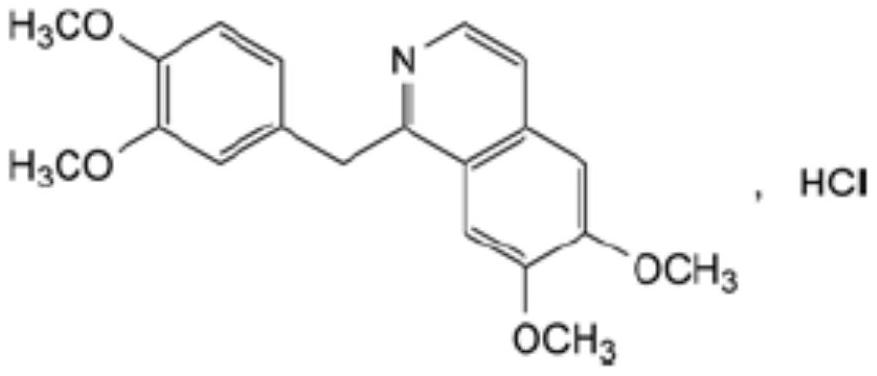
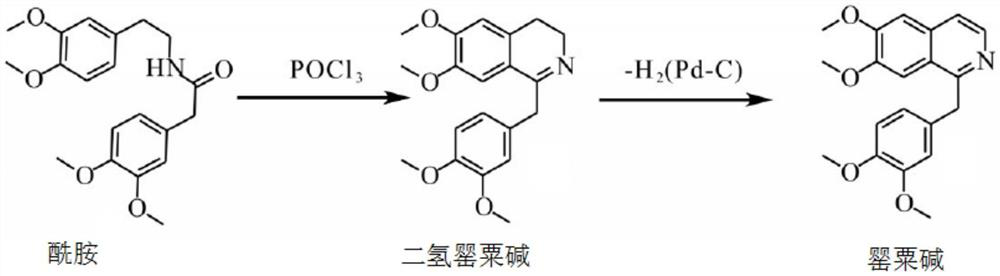
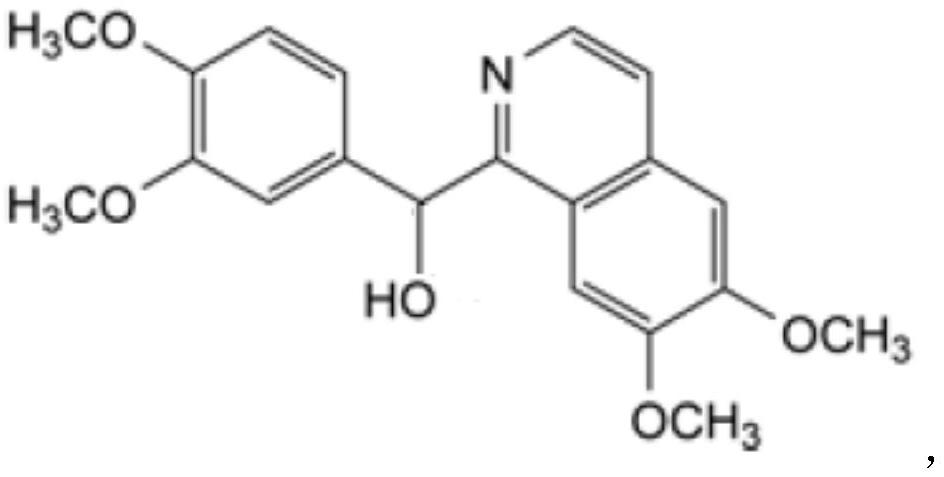
Preparation of powder injection pharmaceutical composition from high-purity papaverine hydrochloride
A technology of papaverine hydrochloride and its mixture, which is applied in the field of medicine and can solve problems such as insignificant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Embodiment 1: Preparation of papaverine hydrochloride powder injection pharmaceutical composition
[0168] formula:
[0169]
[0170]
[0171] Preparation method:
[0172] (a) Take by weighing papaverine hydrochloride and pharmaceutical excipients of the prescribed amount, add an appropriate amount (accounting for 65% of the prescription volume) of water for injection to dissolve, then add 0.5% of the volume of the medicinal liquid with active carbon for needles, stir, filter and decarbonize;
[0173] (b) Add water for injection to the prescribed amount, stir evenly, measure the pH value of the solution and optionally measure the active ingredient content, and adjust to the specified pH value with an acid-base regulator if necessary;
[0174] (c) The medicinal liquid is sterilized and filtered with a microporous membrane, and filled in vials;
[0175] (d) Freeze-drying to remove moisture, and stoppering, to obtain. This powder injection sample can be record...
Embodiment 2
[0176] Embodiment 2: Preparation of papaverine hydrochloride powder injection pharmaceutical composition
[0177] formula:
[0178]
[0179] Preparation method:
[0180] (a) Take by weighing papaverine hydrochloride and pharmaceutical excipients of the prescribed amount, add an appropriate amount (accounting for 50% of the prescription volume) of water for injection to dissolve, then add 0.5% of the volume of the medicinal liquid with active carbon for needles, stir, filter and decarbonize;
[0181] (b) Add water for injection to the prescribed amount, stir evenly, measure the pH value of the solution and optionally measure the active ingredient content, and adjust to the specified pH value with an acid-base regulator if necessary;
[0182] (c) The medicinal liquid is sterilized and filtered with a microporous membrane, and filled in vials;
[0183] (d) Freeze-drying to remove moisture, and stoppering, to obtain. This powder injection sample can be recorded as Ex2. ...
Embodiment 3
[0184] Embodiment 3: Preparation of papaverine hydrochloride powder injection pharmaceutical composition
[0185] formula:
[0186]
[0187]
[0188] Preparation method:
[0189] (a) Take by weighing papaverine hydrochloride and pharmaceutical excipients of the prescribed amount, add an appropriate amount (accounting for 90% of the prescription volume) of water for injection to dissolve, then add 0.8% of the volume of the medicinal liquid with active carbon for needles, stir, and decarbonize by filtration;
[0190] (b) Add water for injection to the prescribed amount, stir evenly, measure the pH value of the solution and optionally measure the active ingredient content, and adjust to the specified pH value with an acid-base regulator if necessary;
[0191] (c) The medicinal liquid is sterilized and filtered with a microporous membrane, and filled in vials;
[0192] (d) Freeze-drying to remove moisture, and stoppering, to obtain. The powder injection sample can be ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com