Self-fused concatenation protein modification method and application thereof
A modification method and protein modification technology, which can be used in the development of self-fusion tandem protein modification methods and the application field of protein modification, which can solve problems such as toxic effects and reducing protein biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
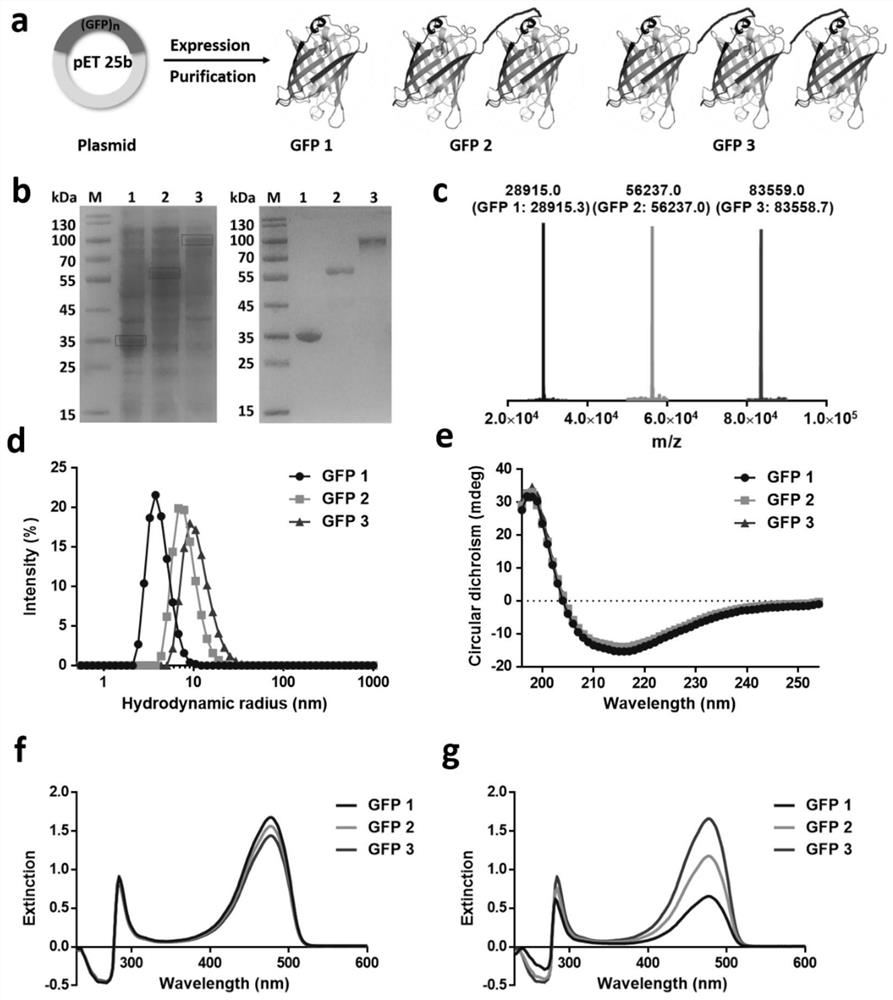
[0029] Biosynthesis and physicochemical property characteristics of embodiment 1 GFP multimer
[0030] Genes encoding GFP monomeric GFP 1, dimeric GFP 2 and trimeric GFP3 (containing 6×His tag) were synthesized by Sangon Biotechnology (Shanghai, China) and successfully cloned into the pET-25b(+) vector . The sequence of the GFP protein is shown in SEQ ID No:1. Multimeric GFP subunits are separated by flexible linkers GGGGS. After confirmation by gene sequencing, the plasmid was transformed into Escherichia coli BL21 (DE3) strain for expression. Before large-scale expression, the transformed monoclonal bacteria were first inoculated in 50 mL TB medium (containing 100 μg / mL ampicillin), and cultured overnight at 37° C. and 250 rpm with shaking. The next day, it was transferred into 1L of fresh TB medium (in a 2L shake flask, the concentration of ampicillin was 100 μg / mL) for large-scale culture and induction of expression. The specific steps are as follows: Firstly, shake cu...
Embodiment 2
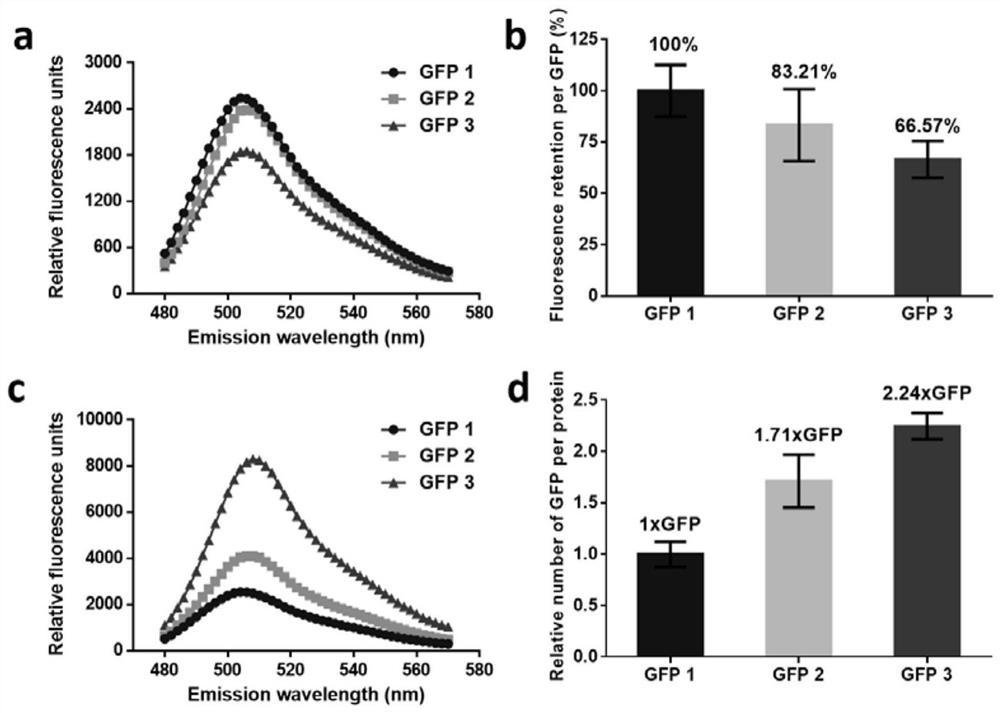
[0036] The biological activity analysis of embodiment 2 GFP multimer
[0037] Protein fluorescence spectrum (480nm-570nm) was analyzed and measured by Varioskan Flash microplate reader (Thermo Scientific, USA), and the excitation wavelength was 460nm. The protein fluorescence value was measured at an excitation wavelength of 460nm and an emission wavelength of 507nm. At the same mass concentration (the protein test concentration is 0.25mg / mL), the retention rate of fluorescence intensity per unit of GFP is obtained by calculation, and the calculation formula is: fluorescence retention rate of multimer per unit of GFP = fluorescence value of multimer / GFP The fluorescence value of 1 x 100%, where the retention rate of GFP fluorescence intensity per unit in GFP 1 is 100%. At the same molar concentration (the protein test concentration is 10 μM), the relative amount of GFP contained in each unit concatemer is obtained by calculation, and the calculation formula is: the amount of ...
Embodiment 3
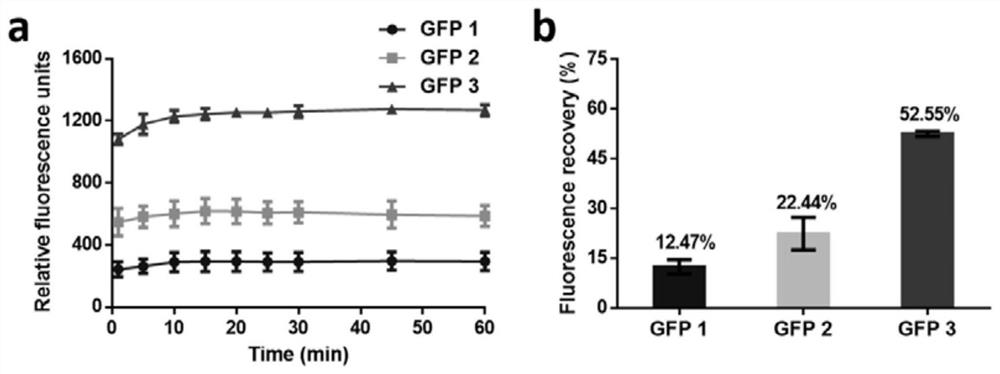
[0039] The in vitro thermal stability analysis of embodiment 3 GFP multimer
[0040] The in vitro thermal stability of the protein was tested by heat-denaturing the protein at 90°C for 2 minutes and refolding at room temperature. Before the test, adjust the fluorescence concentration of the sample to be the same (the final protein fluorescence value measured by a microplate reader is 2500, which is equivalent to the protein concentration of GFP 1, GFP 2 and GFP 3 10, 7 and 5 μM), and the test method is the same as the implementation Example 2. After heating at high temperature for 2 minutes, the protein fluorescence value was measured at given time (1, 5, 10, 15, 20, 25, 30, 45, 60 and 120 min).
[0041] Such as image 3 , GFP 3 recovers fluorescence faster than GFP 2, and GFP 2 recovers fluorescence faster than GFP1 ( image 3 a). After being placed at room temperature for 2 hours, the recovery fluorescence of GFP3 (53%) and GFP2 (22%) was 4.2 times and 1.8 times that of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydrodynamic radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com