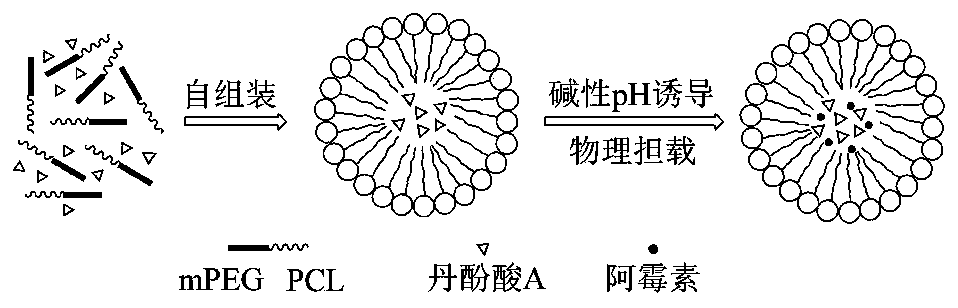
A kind of anthracycline antitumor antibiotic compound micelle and its preparation method and application
An anti-tumor and anthracycline technology, applied in the field of biomedicine, can solve problems such as differences in physical and chemical properties, blanks, etc., and achieve the effects of uniform particle size, high encapsulation efficiency, and sustained drug release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation and characterization of compound nanomicelles loaded with doxorubicin and salvianolic acid A
[0041] Preparation of compound nanomicelles: 20 mg of salvianolic acid A and 100 mg of block copolymer mPEG-PCL were dissolved in 5 mL of acetone, and the organic solvent was removed by rotary evaporation at 55 °C to obtain the polymerization of salvianolic acid A Add 5 mL of ultrapure water to hydrate for 30-60 min, continue to add 0.5 mL of PBS buffer solution (10×) with pH 7.4 and 2 mL of doxorubicin aqueous solution (concentration 3 mg / mL), and stir electromagnetically at room temperature (350 rpm ×20 min) until the solution was completely clarified, and then filtered through a 0.22 μm sterile membrane to obtain compound nanomicelles co-loaded with doxorubicin and salvianolic acid A. At the same time, doxorubicin single-drug micelles without salvianolic acid A were prepared under the same conditions; 3 mg doxorubicin was dissolved in 1 mL ultrapure wa...
Embodiment 2
[0046] Example 2 Preparation of compound nanomicelle freeze-dried powder loaded with doxorubicin and salvianolic acid A
[0047] Dissolve 20 mg salvianolic acid A and 100 mg block copolymer mPEG-PCL in 5 mL acetone, remove the organic solvent by rotary evaporation at 55 °C, add 5 mL ultrapure water, and hydrate for 30-45 min to dissolve the drug film . At the same time, doxorubicin single-drug micelles without salvianolic acid A were prepared under the same conditions. 1–10 mg of doxorubicin was dissolved in 1 mL of ultrapure water to obtain a concentration of doxorubicin of (1–10) mg / mL of doxorubicin in water. Then, continue to add 0.5 mL of PBS buffer solution (10×) at pH 7.4 and 2 mL of doxorubicin aqueous solution (concentration 3 mg / mL), and stir electromagnetically at room temperature (300 rpm×30 min) until the solution is completely clear, and then, Filtration with a 0.22 μm bacteria-removing membrane to obtain compound nanomicelles co-loaded with doxorubicin and sal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com