A kind of irinotecan hydrochloride composition and preparation method thereof
A technology for irinotecan hydrochloride and its composition, which is applied in the field of irinotecan hydrochloride composition and its preparation, can solve the problems of many adverse reactions and limited use, achieve simple and convenient preparation, improve stability, and overcome low encapsulation efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
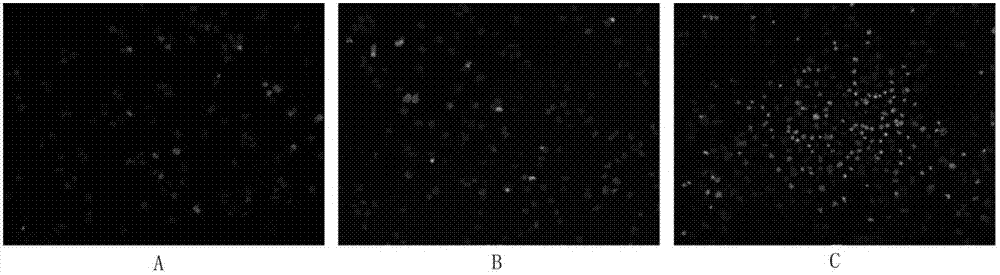
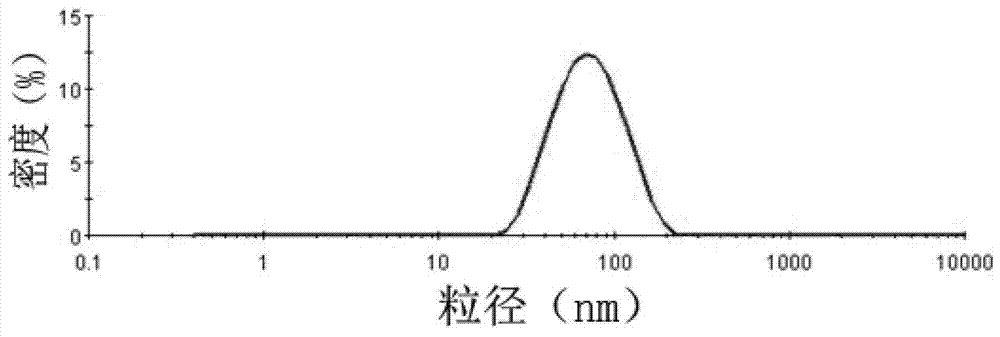
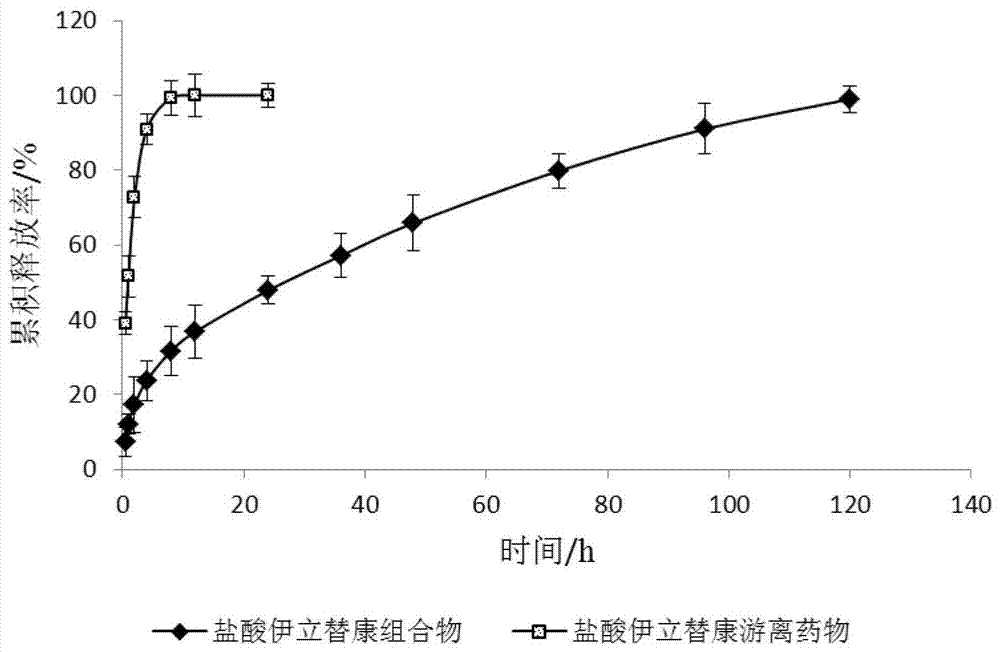
Image
Examples
Embodiment 1
[0045] The preparation of irinotecan hydrochloride composition:
[0046] Dissolve 2g of soybean lecithin, 1g of cholesterol, and 0.5g of PEG-DSPE with 1.5ml of absolute ethanol sonically, inject it into 20ml of 300mM citric acid buffer (pH=3) preheated to 60°C, stir at high speed to obtain the primary product, and then Homogenize under high pressure 4 times at 20,000 psi, filter and sterilize, and dispense into vials of 2 ml / bottle to obtain a blank lipid nano-preparation (phospholipid concentration is 100 mg / ml).
[0047] Weigh 200mg of irinotecan hydrochloride (cpt-11), 9mg of lactic acid, and 450mg of sorbitol, add 20ml of water for injection to dissolve it ultrasonically, adjust the pH to 3-4 with hydrochloric acid (0.1M), and pack in 2ml / bottle in an anatomy , and then sterilized under high pressure at 121°C for 30 minutes to obtain irinotecan hydrochloride solution.
[0048] Prepare sodium carbonate solution (0.5M), filter and sterilize, then pack in 8ml / bottles into vi...
Embodiment 2
[0052] The preparation of irinotecan hydrochloride composition:
[0053] Weigh 2g of HSPC, 0.2g of cholesterol, 0.3g of PEG-DSPE, and 0.3g of RGD-PEG-DSPE and dissolve them in 2ml of absolute ethanol, inject them into 30ml of 300mM copper sulfate solution, stir at high speed, and then dissolve them with polycarbonate with a pore size of 100nm Extrude the membrane for 4 times, then replace the outer aqueous phase with sucrose solution (300mM) by ultrafiltration (molecular weight of the ultrafiltration tube: 10K), filter and sterilize, and divide into vials of 4ml / bottle to obtain the blank fat Quality nano-preparation (phospholipid concentration of 50mg / ml).
[0054] Weigh 200mg of irinotecan hydrochloride (cpt-11), 9mg of lactic acid, and 450mg of sorbitol, add 20ml of water for injection to dissolve it ultrasonically, adjust the pH to 3-4 with hydrochloric acid (0.1M), and pack in 2ml / bottle in an anatomy , and then sterilized under high pressure at 121°C for 30 minutes to o...
Embodiment 3
[0059] The preparation of irinotecan hydrochloride composition:
[0060] Weigh 2g of DSPC, 0.8g of cholesterol, 0.1g of FA-PEG-DSPE, and 0.2g of PEG-DSPE, dissolve them ultrasonically in 1.6ml of absolute ethanol, inject into 40ml of sbe-CD triethylamine solution (250mM), stir at high speed, and then The polycarbonate film of 100nm pore size was extruded 4 times, and the obtained preparation was dialyzed in normal saline for 24h, and after filtering and sterilizing, it was divided into vials at 4ml / bottle to obtain a blank lipid nano-preparation (the concentration of phospholipids was 50mg / ml).
[0061] Weigh 200mg of irinotecan hydrochloride (cpt-11), 9mg of lactic acid, and 450mg of sorbitol, add 20ml of water for injection to dissolve it ultrasonically, adjust the pH to 3-4 with hydrochloric acid (0.1M), and pack in 2ml / bottle in an anatomy , and then sterilized under high pressure at 121°C for 30 minutes to obtain irinotecan hydrochloride solution.
[0062] Prepare sodi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com