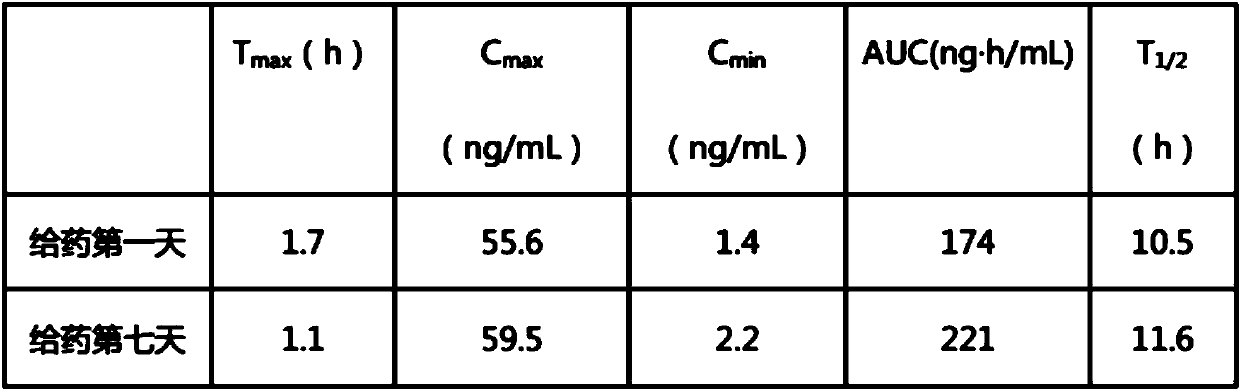
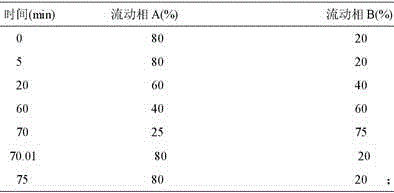
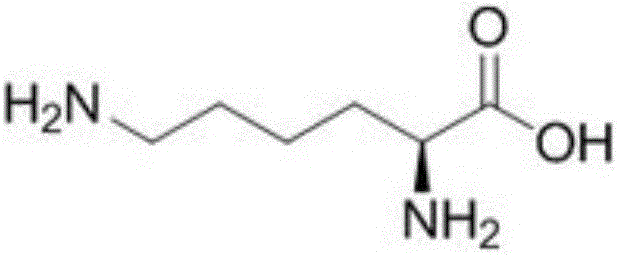
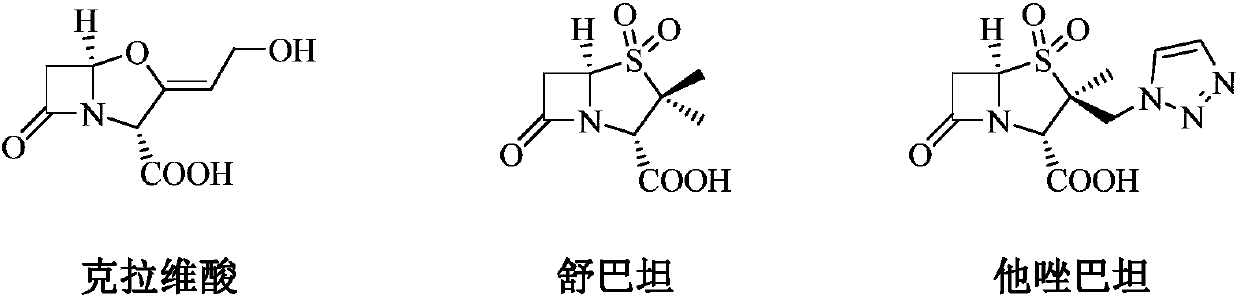
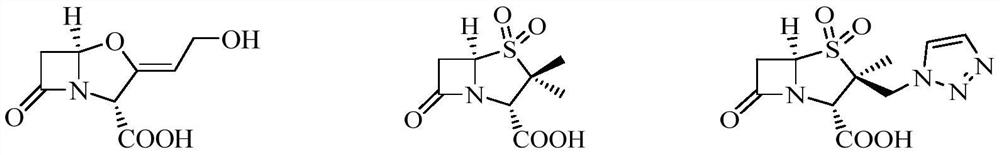
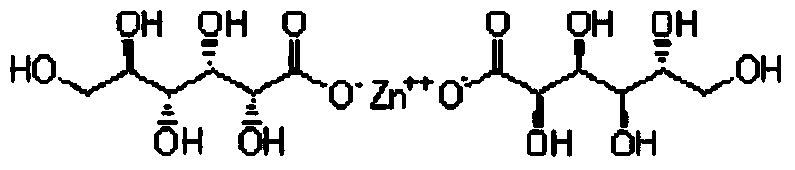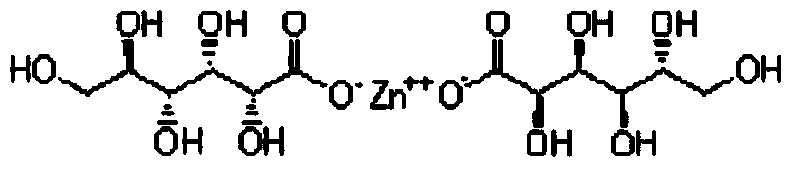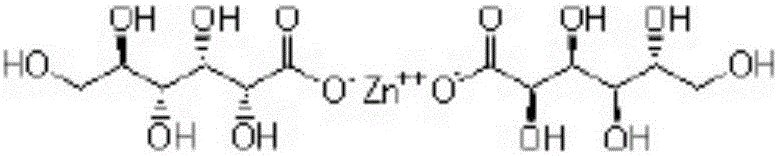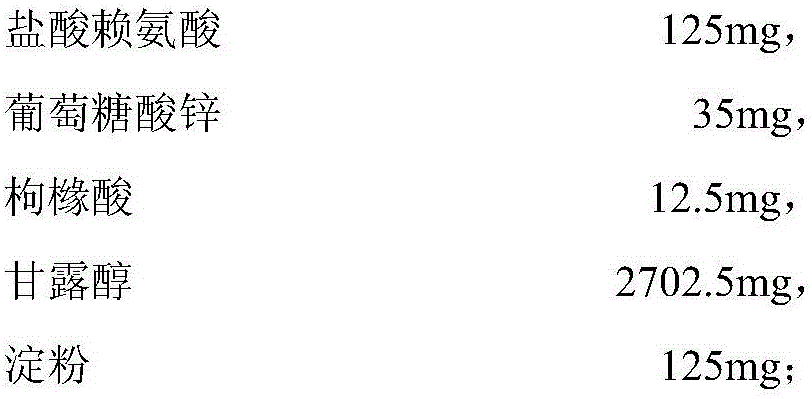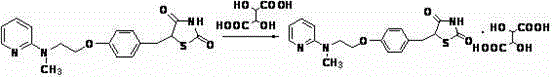Patents
Literature
39results about How to "Improve pharmaceutical properties" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel crystalline form of sitagliptin sulfate
InactiveUS20150037406A1Physical improvementImprove pharmaceutical propertiesBiocideOrganic chemistrySitagliptinSulfate
A novel crystalline form of sitagliptin sulfate is provided. In addition, a method for obtaining the crystalline form, pharmaceutical compositions comprising the novel crystalline form and the crystalline form for use as a medicament are provided.
Owner:MOEHS IBERICA
Compound and crystals thereof
ActiveCN102070618AImprove pharmaceutical propertiesOrganic active ingredientsSenses disorderDiseaseAutoimmune disease
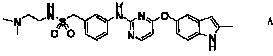
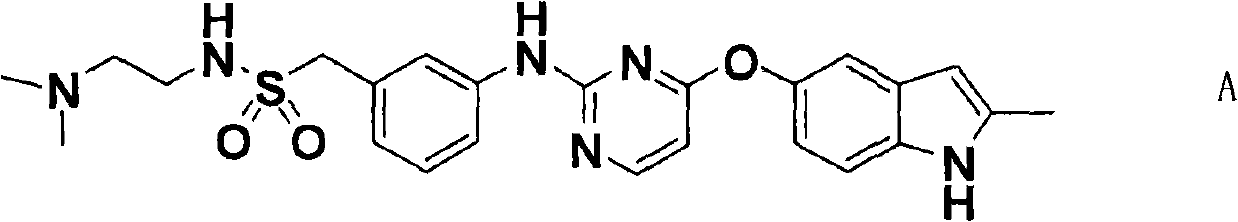
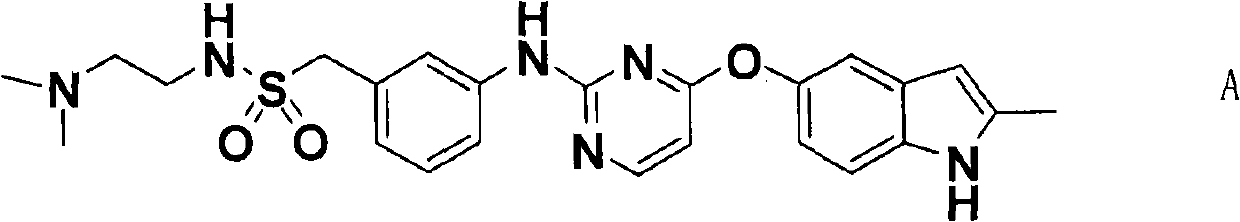
The invention discloses a pyrimidine compound of a structural formula A. The structural formula A is shown below. The pyrimidine compound can be used for preparing medicaments for treating diseases associated with vasculogenesis, in particular medicaments for treating tumor, age-related macular degeneration or autoimmune diseases.
Owner:HUTCHISON MEDIPHARMA LTD
Cisatracurium besilate composition for injection and preparation method and application thereof
ActiveCN104434822AHigh clarityImprove stabilityOrganic active ingredientsPowder deliveryPharmacyMedicine
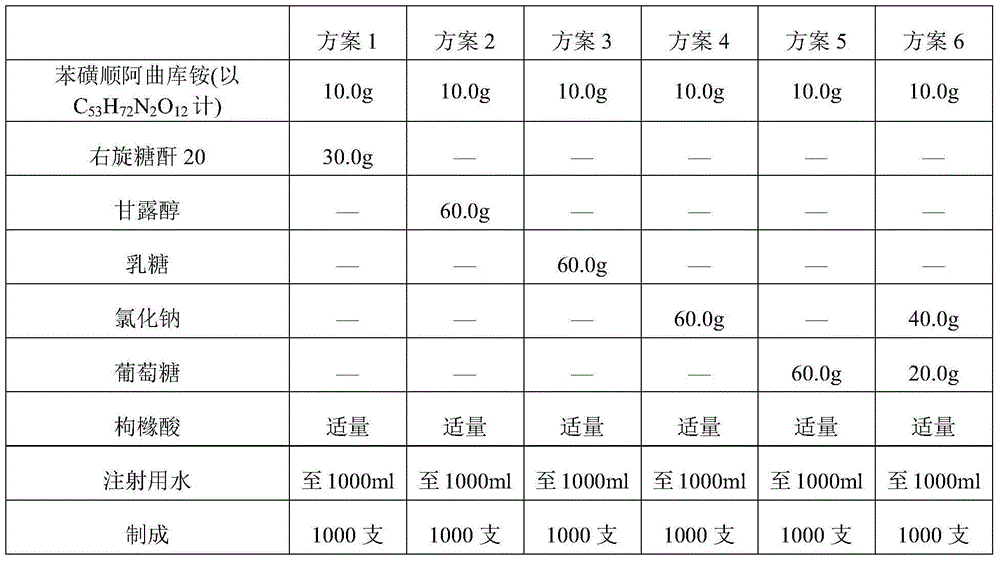
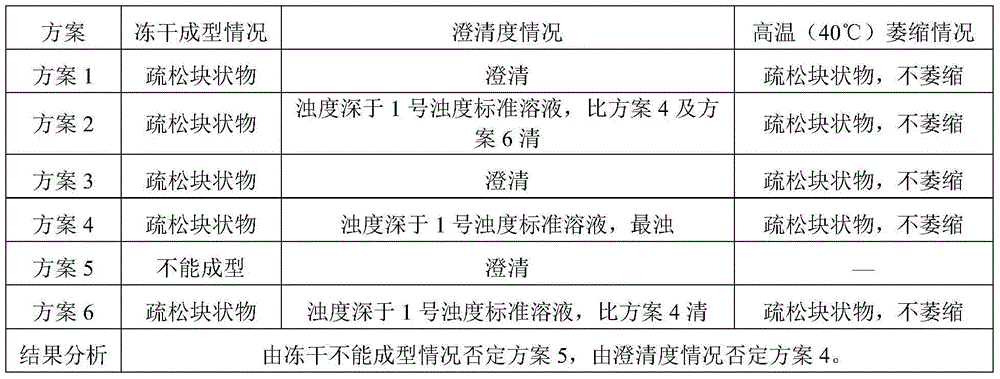
The invention belongs to the field of chemical pharmacy and in particular relates to a cisatracurium besilate composition for injection and a preparation method and application thereof. The cisatracurium besilate composition for injection comprises cisatracurium besilate, sodium chloride, glucose and a pH regulator, wherein cisatracurium besilate takes cisatracurium as an active ingredient, and cisatracurium, sodium chloride and glucose are combined according to a certain ratio. The cisatracurium besilate composition for injection disclosed by the invention is simple in composition, convenient to operate, simple in freeze-drying process, controllable in quality, low in content of product related substances, high in reproducibility and convenient to popularize and utilize.
Owner:HAINAN XIANTONG PHARMA CO LTD
Lurasidone medicine composition and preparation method
InactiveCN102688210AImprove pharmaceutical propertiesPharmaceutical Performance RealizationOrganic active ingredientsNervous disorderLurasidoneWater insoluble
The invention discloses a lurasidone medicine composition and a preparation method. The lurasidone medicine composition comprises the lurasidone shown in the formula (I), sugar alcohol, disintegrant, adhesive, surfactant and optional water-insoluble filler. In the lurasidone medicine composition disclosed by the invention, the contact angle between the surface and water is not greater than 95 degrees. The preparation of lurasidone (particularly tablet) has good release performance and mechanical performance.
Owner:李兴惠
Lurasidone tablet and preparation method thereof
InactiveCN102688209AImprove pharmaceutical propertiesPharmaceutical Performance RealizationOrganic active ingredientsNervous disorderLurasidoneAlcohol sugars
The invention discloses a lurasidone tablet and a preparation method thereof. The lurasidone tablet comprises lurasidone shown in the formula (I), cellulose derivative and sugar alcohol. The prepared lurasidone preparation, especially lurasidone tablet, has good release performance and excellent mechanical performance.
Owner:李兴惠
Complex folic acid/beta-cyclodextrin composite and preparation method thereof
InactiveCN102000345AEasy to manufactureImprove stabilityOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTAqueous solubility
The invention discloses a complex folic acid / beta-cyclodextrin composite and a preparation method thereof. The composite is composed of beta-cyclodextrin and folic acid and the mass ratio of the beta-cyclodextrin to folic acid is 4-7:1. The preparation method comprises the following steps: adding weighted beta-cyclodextrin and folic acid into pure water for mixing to form suspension or solution; under the condition of heating and stirring, standing the reaction solution and cooling the solution until the solid material is sufficiently separated out and filtered; and drying a filter cake after the filtering to obtain the complex folic acid / beta-cyclodextrin composite. The invention has the advantages that the folic acid is embedded in a barrel hollow structure of the beta-cyclodextrin to be the folic acid-beta-cyclodextrin inclusion compound which has obviously enhanced stability, increased solubility and uninfluenced bioactivity; and the active ingredient, namely the folic acid, can be directly applied to foods, medicines and health care products in a clathrate compound mode, thereby overcoming the defects of poor stability, low water-solubility and the like.
Owner:GUANGXI UNIV
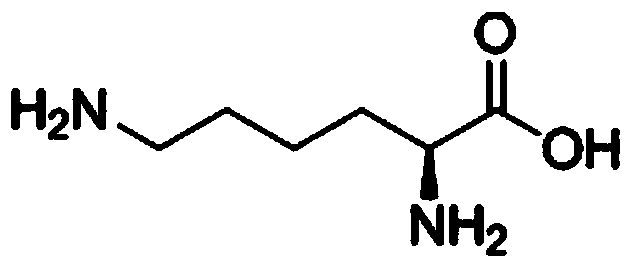
Sugar-free lysine zinc gluconate granules and quality detection method thereof
ActiveCN104173327AImprove pharmaceutical propertiesPrevention of stunted growthOrganic active ingredientsMetabolism disorderGluconic acidIn vivo
The invention relates to sugar-free lysine zinc gluconate granules and a quality detection method thereof, specifically belongs to the technical field of medicine, relates to a composition for supplementing lysine and zinc, especially zinc gluconate, in particular relates to granules for supplementing lysine and zinc, especially zinc gluconate, and more in particular relates to sugar-free lysine zinc gluconate granules for supplementing lysine and zinc, especially zinc gluconate. The invention also relates to a method for performing quality detection on the sugar-free lysine zinc gluconate granules. The lysine zinc gluconate granules are a mineral substance type OTC medicine. The sugar-free lysine zinc gluconate granules are clinically used for preventing and treating growth retardation, malnutrition, anorexia and the like of children and adolescents due to lack of lysine and zinc. The lysine contained in the lysine zinc gluconate granules is one of essential amino acids for maintaining the nitrogen balance of a human body and has the effect of promoting the growth of the human body; zinc is an important component for a plurality of enzymes in vivo, and has the effects of promoting growth and development and improving gustation.
Owner:厦门康艺迈医药科技有限公司
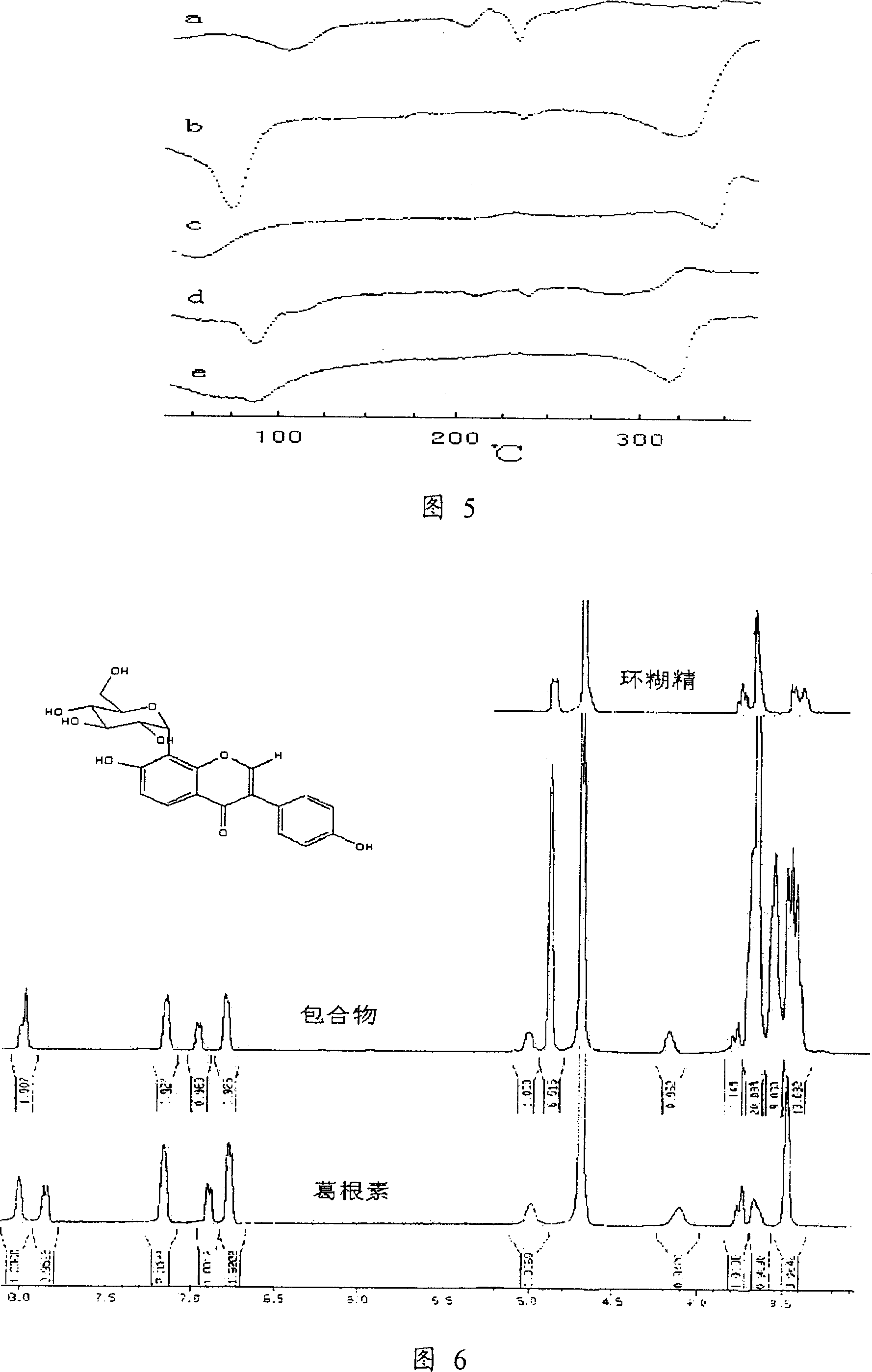
Inclusion puerarin/cyclodextrin composition and its preparing method
InactiveCN1977975AEasy to manufactureHigh dissolution rateOrganic active ingredientsMetabolism disorderOrganic solventPuerarin
The present invention relates to an inclusion state puerarin / cyclodextrin composition in which the mole ratio of cyclodextrin and puerarin is 1-5:1. the described cyclodextrin can be beta-cyclodextrin or hydroxypropyl-beta-cyclodextrin or sulfo-bulyl-beta-cyclodextrin or their mixture. Besides, said invention also provides its preparation method and concrete steps.
Owner:NANJING NORMAL UNIVERSITY +1
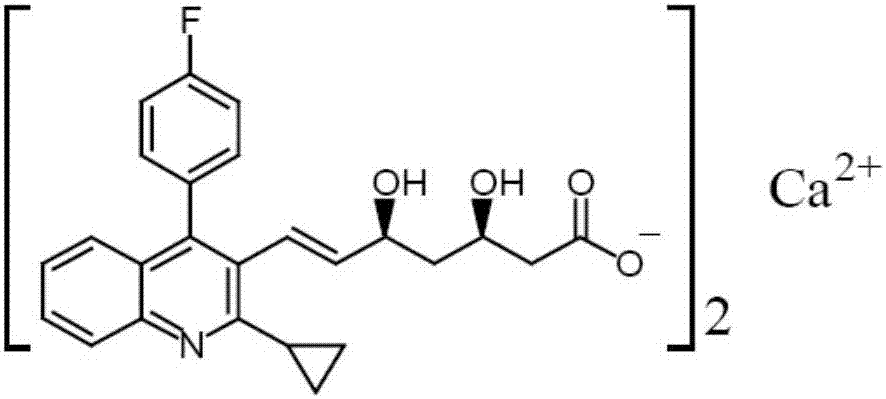
Pitavastatin calcium tablet pharmaceutical composition and dry or wet preparation method thereof
ActiveCN107126423AGood content uniformityReduce volumeMetabolism disorderPharmaceutical non-active ingredientsAluminum magnesium silicateCholesterol blood
The invention relates to a pitavastatin calcium tablet pharmaceutical composition and a dry or wet preparation method thereof. Specifically, one aspect of the invention relates to a pitavastatin calcium tablet which comprises a tablet core and a coating layer, wherein the tablet core comprises 1 part of pitavastatin calcium, 40-120 parts of a filling agent, 4-20 parts of a disintegrating agent, 0.5-5 parts of an adhesive, 0.5-5 parts of a stabilizer and 0.3-3 parts of a lubricating agent. The tablet core can be made from lactose, microcrystalline cellulose, tricalcium phosphate, low substituted hydroxypropy cellulose, crospovidone, sodium carboxymethyl starch, aluminum-magnesium silicate, aluminum-magnesium metasilicate, hydroxypropyl methylcellulose, magnesium stearate and other excipients and combinations thereof. The tablet core can be prepared through a dry granulation tabletting process or a wet granulation tabletting process. The invention further relates to a preparation method of pitavastatin calcium tablets and application thereof in preparation of drugs for treating and / or preventing hypercholesteremia or familial hypercholesterolemia. The tablet pharmaceutical composition disclosed by the invention has excellent properties shown in the specification.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
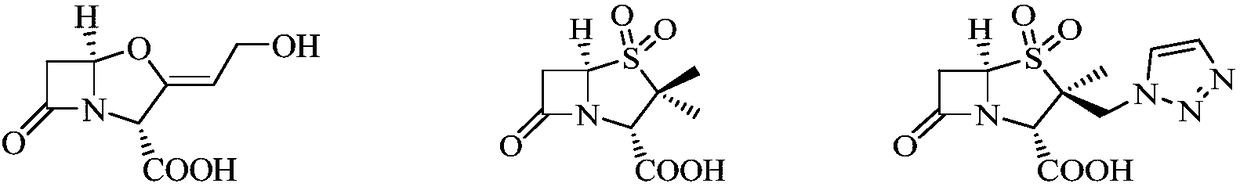
Antibacterial composition and uses thereof
ActiveCN108619141AHigh antibacterial activityLow inhibitory concentrationAntibacterial agentsOrganic active ingredientsAntibiotic YSolvent
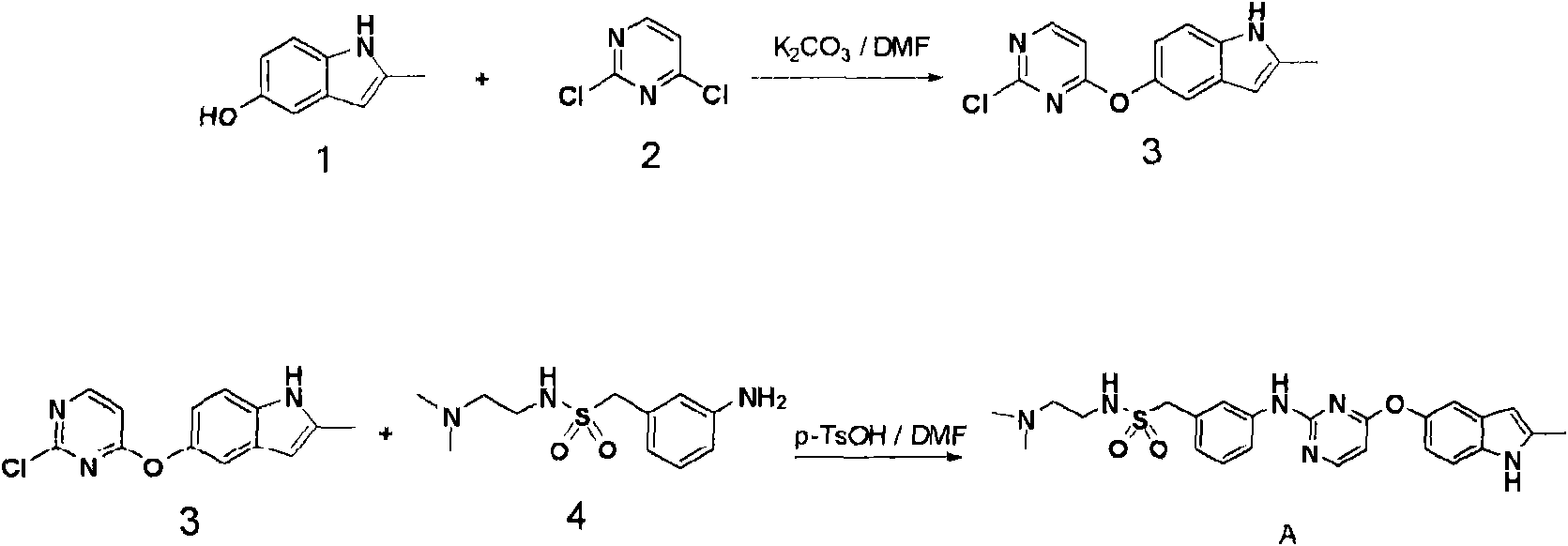
The present invention relates to the technical field of medicine, specifically to a composition, which contains a compound (a), a pharmaceutically acceptable salt, an ester, a solvate or a stereoisomer thereof, and at least a carbapenem antibiotic or a derivative thereof, wherein the compound (a) has a structure represented by a formula (I) defined in the specification. The invention further relates to uses of the composition in preparation of drugs for prevention and / or treatment of infectious diseases caused by bacteria, wherein preferably the bacteria have drug resistance caused by beta-lactamase.
Owner:SHANDONG XUANZHU PHARMA TECH CO LTD
Compound and crystals thereof
ActiveCN102070618BImprove pharmaceutical propertiesOrganic active ingredientsSenses disorderMedicinePharmaceutical Substances
Owner:HUTCHISON MEDIPHARMA LTD
Sugar-free lysine and gluconic acid zinc granule composition and preparation method thereof
ActiveCN104161746AImprove pharmaceutical propertiesPrevention of stunted growthOrganic active ingredientsMetabolism disorderChemistrySugar free
The invention relates to a sugar-free lysine and gluconic acid zinc granule composition and a preparation method thereof, belongs to the technical field of the medicine, relates to a composition for supplementing lysine and zinc, and especially gluconic acid zinc, in particular relates to a granule for supplementing the lysine and the zinc, and especially the gluconic acid zinc, and more specifically relates to a sugar-free granule for supplementing the lysine and the zinc, and especially the gluconic acid zinc. The invention also relates to a method for preparing the composition. The lysine and gluconic acid zinc granule is a mineral substance type nonprescription drug. The lysine and gluconic acid zinc granule is clinically used for preventing and treating growth retardation, malnutrition, anorexia and the like of children and adolescents for lack of lysine and zinc. The lysine contained in the lysine and gluconic acid zinc granules is one of the necessary amino acids for maintaining the nitrogen balance of a human body and is capable of promoting the growth of the human body; the zinc is an important component of a plurality of enzymes in vivo and is capable of promoting the growth and improving the sense of taste.
Owner:厦门康艺迈医药科技有限公司
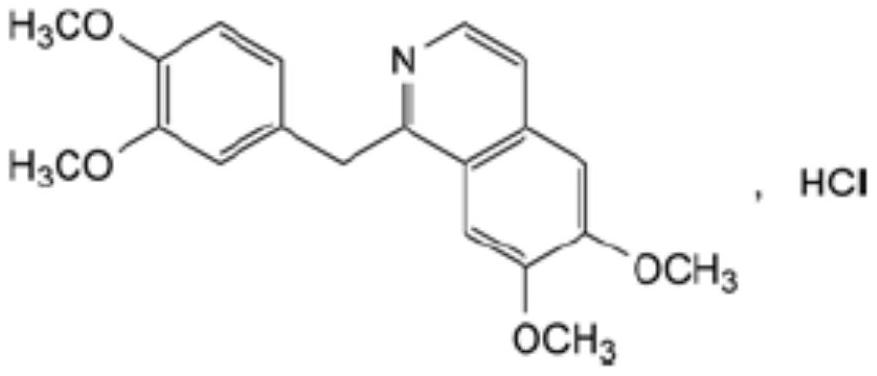
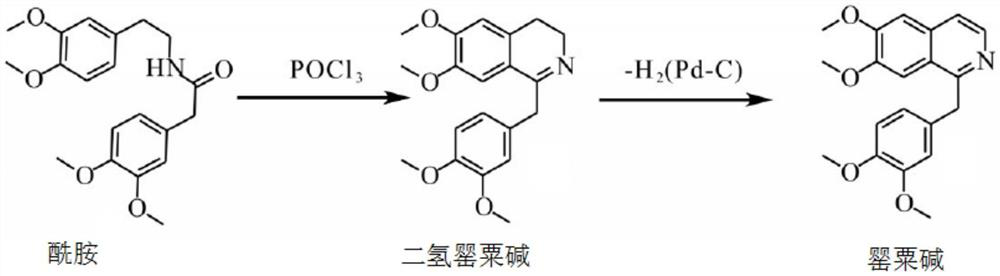
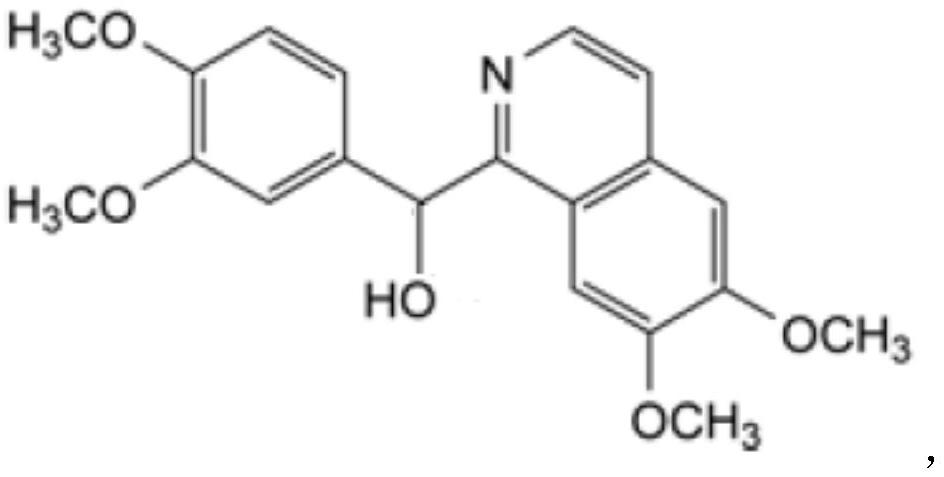
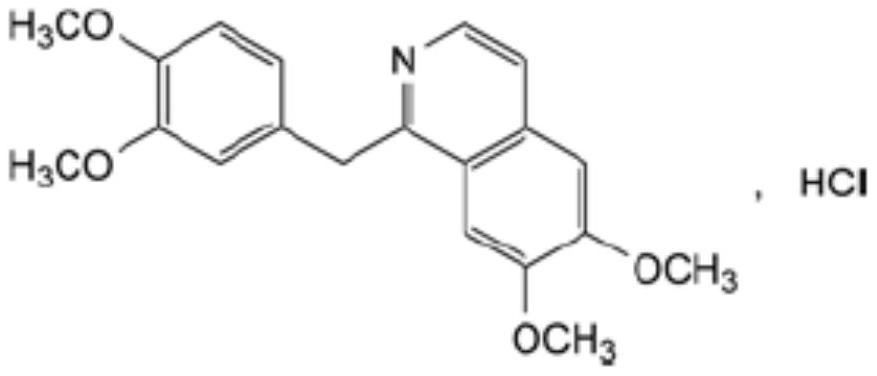
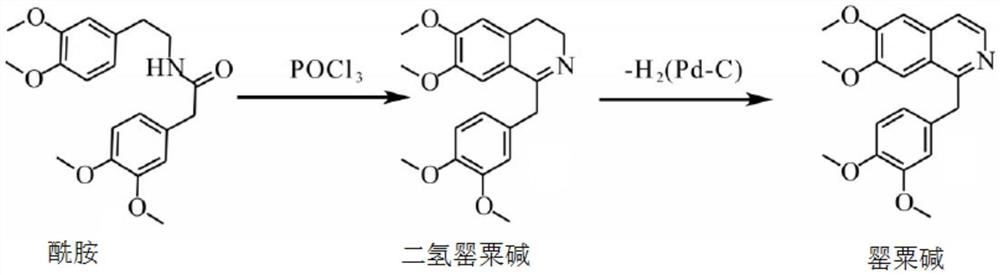
Preparation of powder injection pharmaceutical composition from high-purity papaverine hydrochloride
ActiveCN111848512AImprove pharmaceutical propertiesOrganic active ingredientsPowder deliveryPtru catalystChlorethoxyfos
The invention relates to preparation of a powder injection pharmaceutical composition from high-purity papaverine hydrochloride, in particular to a preparation method of papaverine hydrochloride. Themethod comprises the following steps of: heating 3, 4-dimethoxy-beta-phenyl-ethylamine and 3, 4-dimethoxy-phenyl-acetic acid to melting, and then carrying out reaction in a mixture of benzene and chlorethoxyfos to obtain 6, 7, 3', 4'-tetramethoxy-1-benzyl-dihydro-isoquinoline hydrochloride; then dissolving the wet product in tetrahydronaphthalene after the wet product becomes free alkali, and carrying out dehydrogenation reaction at 180DEG C in the presence of a Raney nickel catalyst; after dehydrogenation is finished, directly filtering the tetrahydronaphthalene reaction mixture from the Raney nickel catalyst into a mixture of a hydrochloric acid aqueous solution and methanol; filtering out precipitates, and performing recrystallizing from the ethanol-water solution in an inert gas environment to obtain off-white 6, 7, 3', 4'-tetramethoxy-1-benzyl isoquinoline hydrochloride, namely papaverine hydrochloride. The invention also relates to a papaverine hydrochloride powder injection pharmaceutical composition, and a preparation method and a quality detection method thereof. The invention achieves excellent technical effects as described in the specification.
Owner:山东北大高科华泰制药有限公司
Papaverine hydrochloride powder injection for injection and quality detection method thereof
ActiveCN111796043AImprove pharmaceutical propertiesPowder deliveryOrganic active ingredientsO-Phosphoric AcidGradient elution
The invention relates to a papaverine hydrochloride powder injection for injection and a quality detection method thereof, in particular to a method for determining the content of impurities A, B, C,D, E and F in the powder injection by using a high performance liquid chromatography. The method comprises the following steps that determination is carried out according to the specification of '0512high performance liquid chromatography' in the fourth part of Chinese Pharmacopoeia 2015 edition; an Eclipse XDB-C8 column is used as a chromatographic column, and mobile phases as follows are adopted: the mobile phase A is 3.4 g / L monopotassium phosphate solution of which the pH is adjusted to 3.0 by dilute phosphoric acid, the mobile phase B is acetonitrile, and the mobile phase C is methanol;a gradient elution program is adopted, and the detection wavelength is 238 nm; and in a system suitability test, the separation degree between an impurity A peak and a papaverine peak is not less than1.5. The invention also relates to a papaverine hydrochloride powder injection for injection. The method and the papaverine hydrochloride powder injection for injection have excellent technical effects as shown in the specification.
Owner:山东北大高科华泰制药有限公司
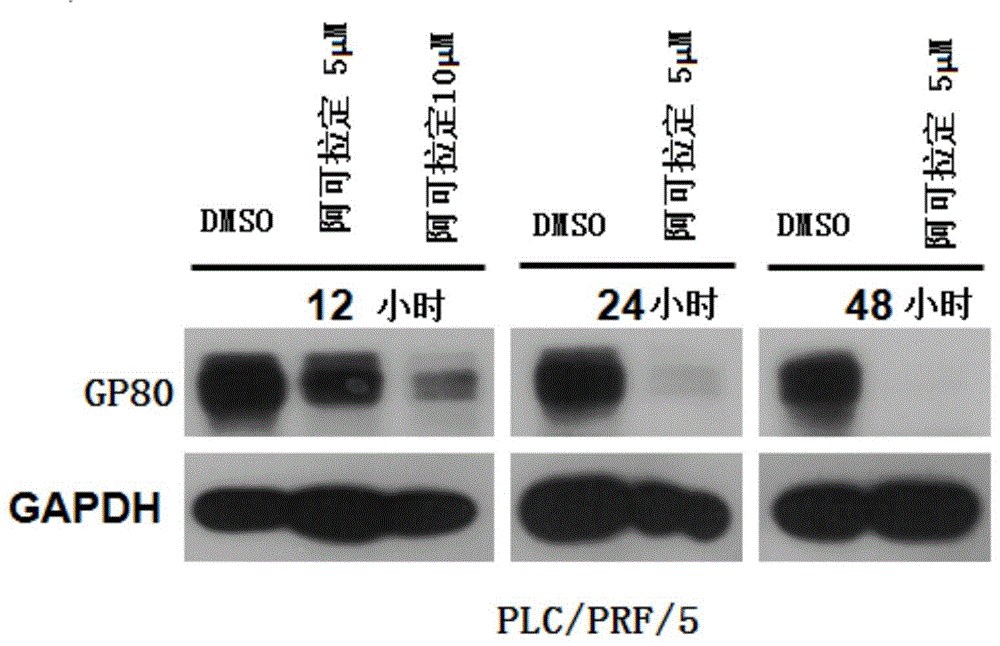
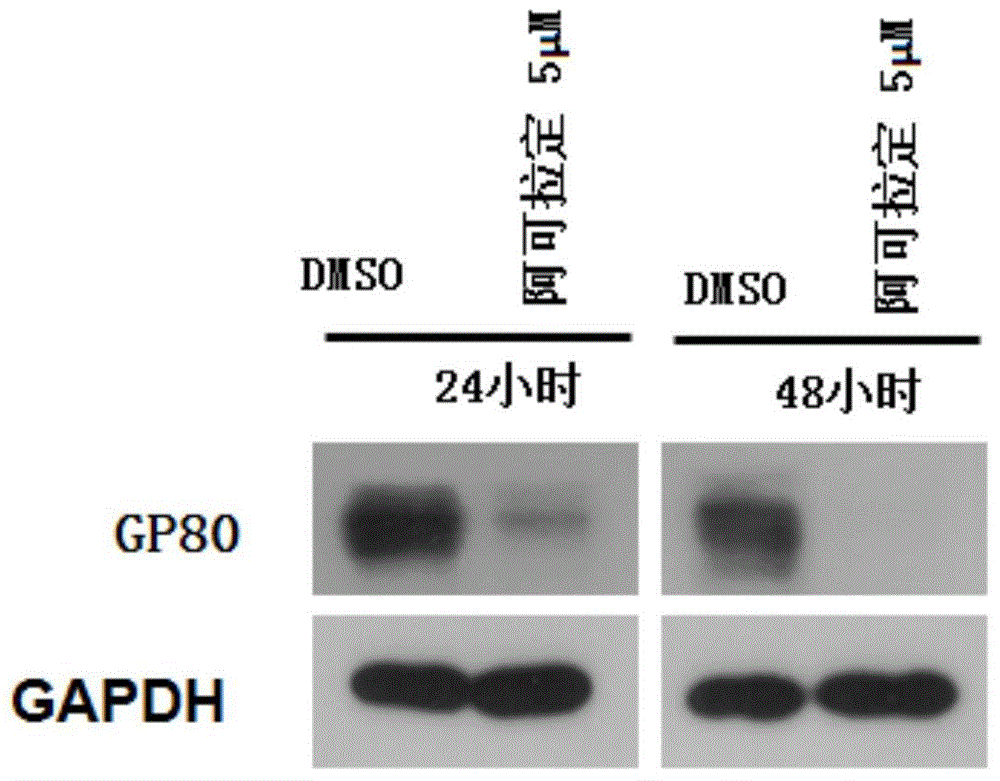
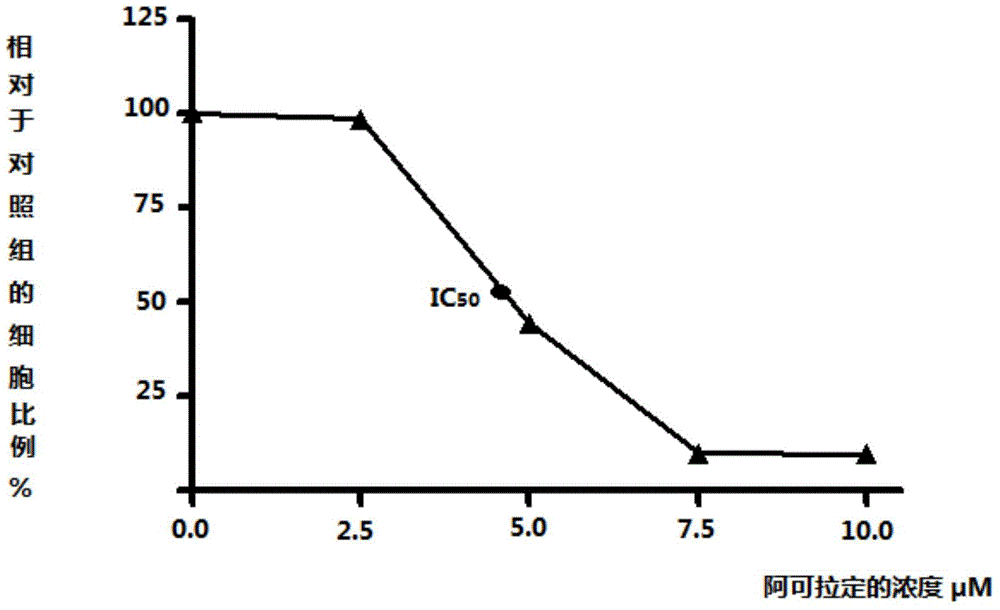
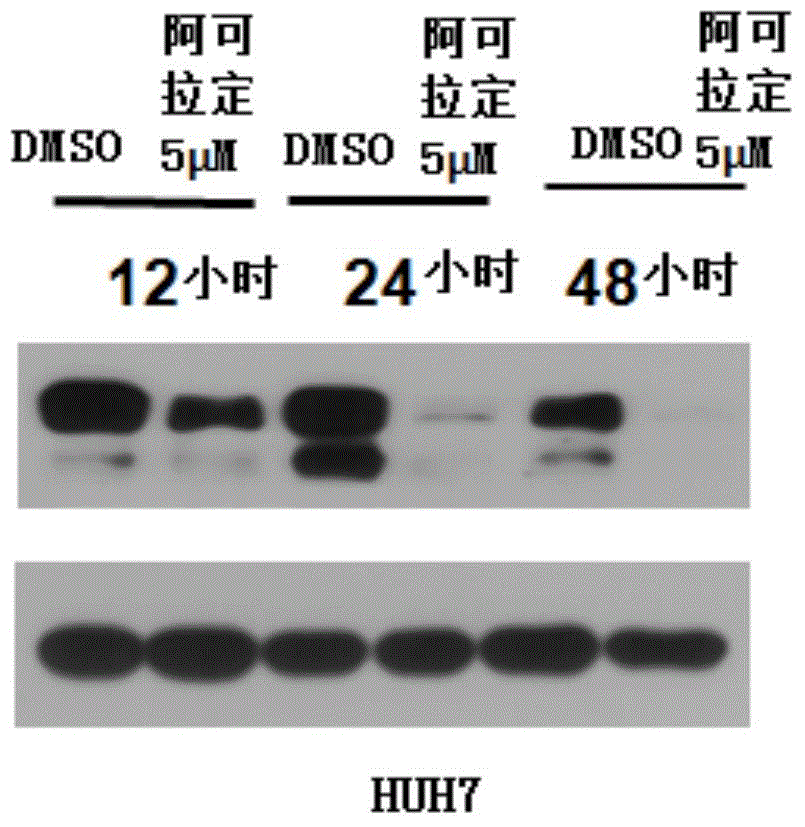
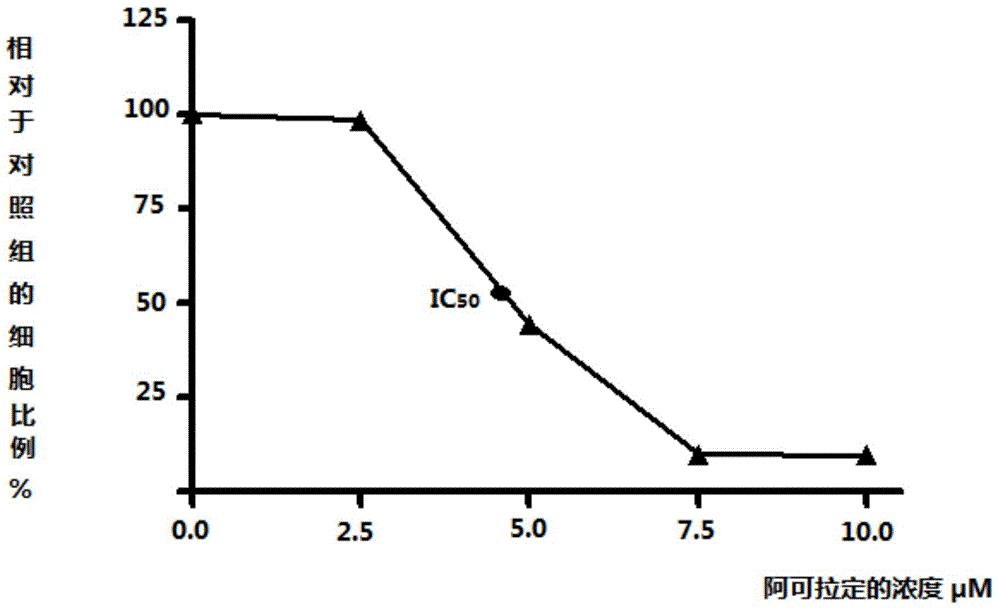
Use of icaritin in preparation of drugs for treating GP80 related diseases
InactiveCN106138027AGood pharmaceutical effectGood effectOrganic active ingredientsDigestive systemDrugLiver cancer
The invention relates to the use of alcladinine in the preparation of medicines for diseases related to GP80, especially for liver cancer related to GP80, alcladine has shown good pharmaceutical effects. The present invention provides a further research direction for the targeting of alcradine in the treatment of cancer.
Owner:BEIJING SHENOGEN PHARMA GRP
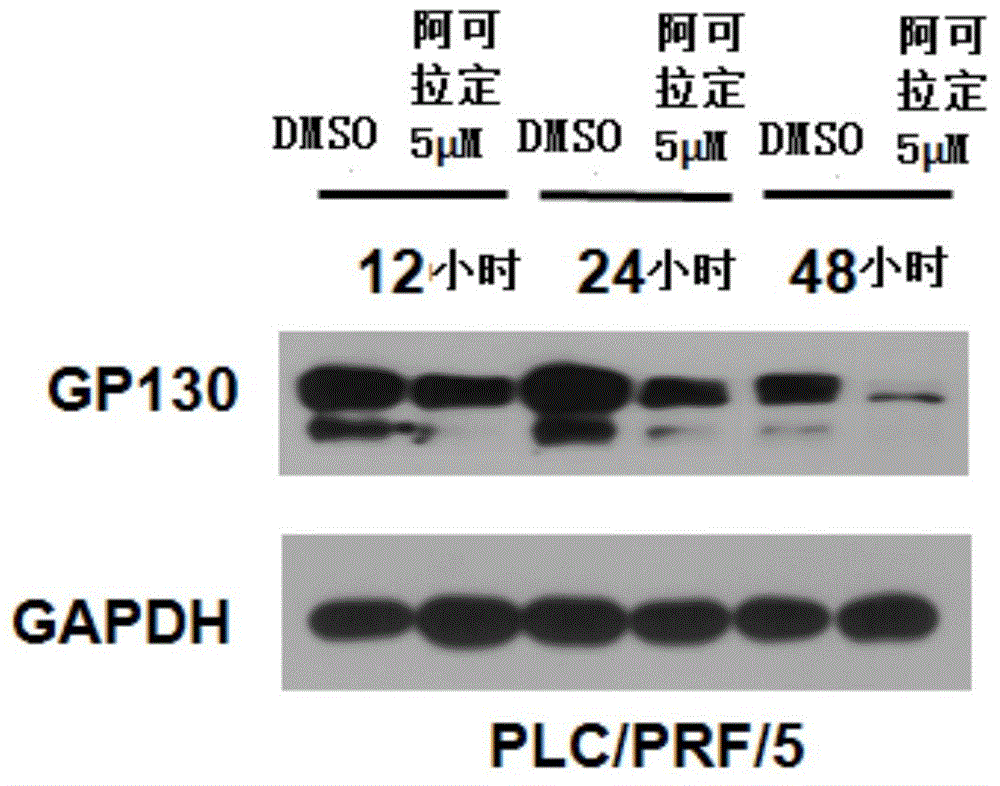
Application of Icaritin in preparation of drugs for curing GP130 related diseases
InactiveCN106138028AGood pharmaceutical effectGood effectOrganic active ingredientsDigestive systemDrugLiver cancer
The invention relates to application of Icaritin in preparation of drugs for curing GP130 related diseases, particularly GP130 related liver cancer, and the Icaritin shows excellent pharmaceutical effect. According to the application of the Icaritin in preparation of the drugs for curing the GP130 related diseases, research direction is further provided for Icaritin in targeting of cancer curing.
Owner:BEIJING SHENOGEN PHARMA GRP
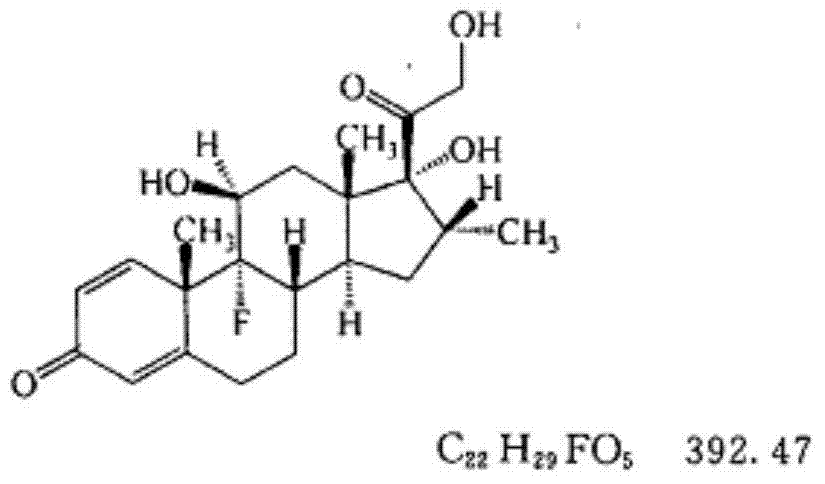
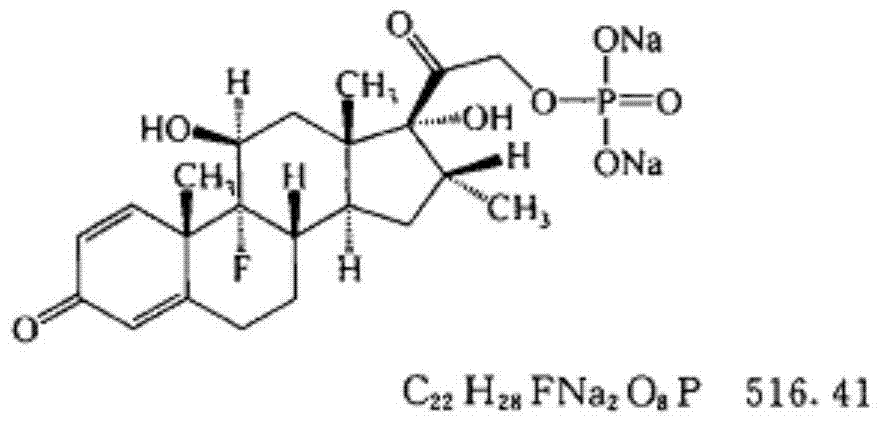
Pharmaceutical composition of dexamethasone sodium phosphate for injection and preparation method of pharmaceutical composition
ActiveCN104490796AMeet storage requirementsImprove pharmaceutical propertiesPowder deliveryOrganic active ingredientsMalignant lymphomaAutoimmune responses
The invention relates to a pharmaceutical composition of dexamethasone sodium phosphate for injection and a preparation method of the pharmaceutical composition, and in particular relates to a freeze-dried powder injection pharmaceutical composition of dexamethasone sodium phosphate. The freeze-dried powder injection pharmaceutical composition comprises dexamethasone sodium phosphate and a freeze-dried excipient which is selected from one or more of mannitol, glycine, lactose and saccharose, wherein the weight ratio of dexamethasone sodium phosphate to mannitol is 1:(1-50). The freeze-dried powder injection pharmaceutical composition of dexamethasone sodium phosphate disclosed by the invention is applicable to treatment of irritability and autoimmunity-related diseases, is particularly used for connective tissue diseases, active rheumatism, rheumatoid arthritis, lupus erythematosus, severe bronchial asthma, severe dermatitis, ulcerative colitis, acute leukemia and the like, and also can be used for comprehensive treatment on serious infection and poisoning, and malignant lymphoma. The freeze-dried powder injection pharmaceutical composition of dexamethasone sodium phosphate disclosed by the invention has excellent pharmaceutical property as described in the specification.
Owner:成都天台山制药股份有限公司
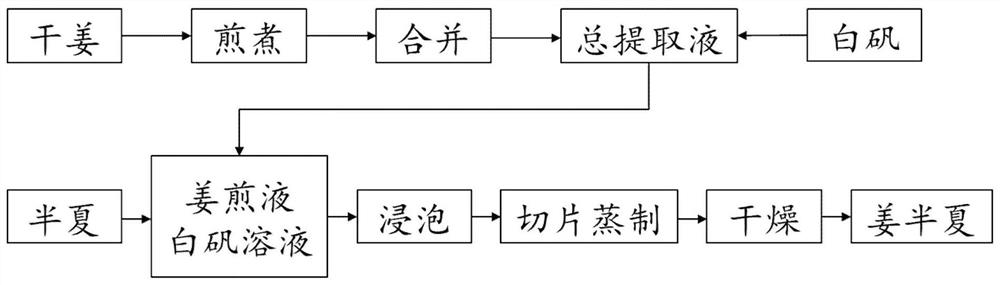
Processing method of ginger processed pinellia
PendingCN113197957AHigh extract contentReduce dosageRespiratory disorderAluminium/calcium/magnesium active ingredientsGinger RhizomeZingiber cassumunar
The invention is applicable to the technical field of processing of traditional Chinese medicine decoction pieces, and provides a processing method of ginger processed pinellia, which comprises the following steps: adding water into dried ginger, decocting twice, respectively collecting and combining a first extracting solution and a second extracting solution to obtain a total extracting solution, adding alum into the total extracting solution, stirring and dissolving to obtain a ginger decoction and alum solution, and drying to obtain the ginger processed pinellia; soaking pinellia ternate in the ginger decoction and the alum solution, taking out, slicing, steaming and drying to obtain ginger pinellia ternate; processing the rhizoma pinelliae by adopting the dried ginger, and the matching of auxiliary materials, a soaking process and a process of slicing first and then steaming are optimized, so that the content of the obtained extract is remarkably increased and reaches 21.82%, the contrast amplification is 29.97%, and the limit of alum is 5.735%; the dried ginger is small in dosage, easy to store and suitable for large-scale production; the prepared ginger processed pinellia is brown to brown and meets the regulations of Chinese Pharmacopoeia; the whole process is few in procedures, simple in equipment and low in energy consumption, water treatment time is shortened, effective components are reserved as far as possible, alum is properly reserved, and the medicinal effect of ginger processed pinellia is improved.
Owner:山西元和堂中药有限公司
Rhein derivative and antiviral application thereof
ActiveCN112920141AStrong inhibitory activityImprove pharmaceutical propertiesOrganic chemistryAntiviralsNew medicationsAntiviral drug
The invention relates to a rhein derivative and an antiviral application thereof, and belong to a new drug development technology. The rhein derivative has a structure as shown in a general formula (I) or a formula (II), and experimental data show that the rhein derivative has better antiviral drug activity.
Owner:CHINA INSPECTION SCI PHARM BEIJING GRP CO LTD
Orally disintegrating tablet pharmaceutical composition comprising tamsulosin and dutasteride
ActiveCN108066347BImprove pharmaceutical propertiesHigh hardnessInorganic non-active ingredientsPill deliveryOrally disintegrating tabletTamsulosin
The present invention relates to an orally disintegrating tablet pharmaceutical composition comprising tamsulosin and dutasteride. It specifically relates to an orally disintegrating tablet, which is a tablet compressed by a tableting process; the tablet includes a tablet matrix composed of various auxiliary materials, and two One kind of coated pellets; the two kinds of coated pellets respectively include a pellet core containing two different active ingredients and at least one layer of coating covering the surface of the pellet core. The invention also relates to a preparation method of the orally disintegrating tablet and its corresponding use. The orally disintegrating tablet of the present invention exhibits the excellent technical effect described in the description of the present invention.
Owner:SHENZHEN WANHE PHARMA
Pitavastatin calcium tablet pharmaceutical composition and its dry or wet preparation method
ActiveCN107126423BImprove pharmaceutical propertiesGood chemical stabilityMetabolism disorderPharmaceutical non-active ingredientsFamilial hypercholesteremiaCholesterol
The invention relates to a pitavastatin calcium tablet pharmaceutical composition and a dry or wet preparation method thereof. Specifically, one aspect of the invention relates to a pitavastatin calcium tablet which comprises a tablet core and a coating layer, wherein the tablet core comprises 1 part of pitavastatin calcium, 40-120 parts of a filling agent, 4-20 parts of a disintegrating agent, 0.5-5 parts of an adhesive, 0.5-5 parts of a stabilizer and 0.3-3 parts of a lubricating agent. The tablet core can be made from lactose, microcrystalline cellulose, tricalcium phosphate, low substituted hydroxypropy cellulose, crospovidone, sodium carboxymethyl starch, aluminum-magnesium silicate, aluminum-magnesium metasilicate, hydroxypropyl methylcellulose, magnesium stearate and other excipients and combinations thereof. The tablet core can be prepared through a dry granulation tabletting process or a wet granulation tabletting process. The invention further relates to a preparation method of pitavastatin calcium tablets and application thereof in preparation of drugs for treating and / or preventing hypercholesteremia or familial hypercholesterolemia. The tablet pharmaceutical composition disclosed by the invention has excellent properties shown in the specification.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Sugar-free lysine zinc glucose granule and its quality detection method
ActiveCN104173327BImprove pharmaceutical propertiesPrevention of stunted growthOrganic active ingredientsMetabolism disorderGluconic acidIn vivo
The invention relates to sugar-free lysine zinc gluconate granules and a quality detection method thereof, specifically belongs to the technical field of medicine, relates to a composition for supplementing lysine and zinc, especially zinc gluconate, in particular relates to granules for supplementing lysine and zinc, especially zinc gluconate, and more in particular relates to sugar-free lysine zinc gluconate granules for supplementing lysine and zinc, especially zinc gluconate. The invention also relates to a method for performing quality detection on the sugar-free lysine zinc gluconate granules. The lysine zinc gluconate granules are a mineral substance type OTC medicine. The sugar-free lysine zinc gluconate granules are clinically used for preventing and treating growth retardation, malnutrition, anorexia and the like of children and adolescents due to lack of lysine and zinc. The lysine contained in the lysine zinc gluconate granules is one of essential amino acids for maintaining the nitrogen balance of a human body and has the effect of promoting the growth of the human body; zinc is an important component for a plurality of enzymes in vivo, and has the effects of promoting growth and development and improving gustation.
Owner:厦门康艺迈医药科技有限公司
Antibacterial combination and application thereof
ActiveCN109568323AHigh antibacterial activityLow inhibitory concentrationAntibacterial agentsOrganic active ingredientsDrugSolvent
The invention relates to the technical field of medicines, in particular to a medicine product. The medicine product comprises a compound (a), or salt or ester or a solvent compound, which can be pharmaceutically accepted, of the compound (a), or stereoisomer of the compound (a), and one or more beta-lactam antibiotics or derivatives of the beta-lactam antibiotics, wherein the compound (a) has a structure shown in a formula (I) (please see the specifications for the formula (I)). Meanwhile, the invention further relates to application of the medicine product to preparation of medicines for treating and / or preventing bacterial infectious diseases, preferably, bacteria have medicine resistance caused by the beta-lactamase.
Owner:BEIJING AOHE DRUG RES INST
A kind of antibacterial composition and its application
ActiveCN108619141BHigh antibacterial activityLow inhibitory concentrationAntibacterial agentsOrganic active ingredientsPharmaceutical medicinePerylene derivatives
The present invention relates to the field of medical technology, and specifically designs a composition comprising compound (a), its pharmaceutically acceptable salt, its ester, its solvate or its stereoisomer, and at least one carbapenem antibiotics or derivatives thereof, wherein the compound (a) has the structure shown in formula (I). The present invention also relates to the use of the composition for preventing and / or treating infectious diseases caused by bacteria, preferably, the bacteria have drug resistance caused by β-lactamase.
Owner:SHANDONG XUANZHU PHARMA TECH CO LTD
Sugar-free zinc lysine granule composition and preparation method
ActiveCN104161746BImprove pharmaceutical propertiesPrevention of stunted growthOrganic active ingredientsMetabolism disorderMedicineEssential amino acid
The invention relates to a sugar-free zinc lysine granule composition and a preparation method. The invention belongs to the technical field of medicine, relates to a composition for supplementing lysine and zinc, especially zinc gluconate, in particular to a granule for supplementing lysine and zinc, especially zinc gluconate, more particularly The invention relates to a sugar-free granule for supplementing lysine and zinc, especially zinc gluconate. The invention also relates to a method for preparing said composition. The zinc lysine granule provided by the invention is a mineral non-prescription medicine. It is clinically used to prevent growth retardation, malnutrition and anorexia caused by lack of lysine and zinc in children and adolescents. Lysine Glucose Zinc Granules contain lysine, which is one of the essential amino acids to maintain the nitrogen balance of the human body, and has the effect of promoting human growth; zinc is an important component of various enzymes in the body, which can promote growth and improve taste.
Owner:厦门康艺迈医药科技有限公司
Clindamycin Phosphate Injection Pharmaceutical Composition and Preparation Method
ActiveCN104095809BImprove pharmaceutical propertiesAntibacterial agentsOrganic active ingredientsDrugBacteriostatic agent
The invention relates to a pharmaceutical composition and a preparation method of clindamycin phosphate injection. Specifically, the present invention belongs to the field of medical technology, and relates to a pharmaceutical composition for injection and a preparation process thereof, in particular to a pharmaceutical composition for clindamycin injection and a preparation method thereof, more particularly to a Clindamycin phosphate injection pharmaceutical composition and its preparation method. In one embodiment, the pharmaceutical composition of clindamycin phosphate injection of the present invention comprises clindamycin phosphate, complexing agent, bacteriostat, acid-base regulator and water for injection. The pharmaceutical composition of clindamycin phosphate injection for injection of the present invention has remarkable and excellent pharmaceutical properties.
Owner:CHENGDU TIANTAISHAN PHARMA
A pharmaceutical composition for treating gynecological inflammation, its preparation method and application
ActiveCN106580981BImprove the effect of anti-infection treatmentHigh cure rateAntibacterial agentsOrganic active ingredientsAdditive ingredientCiclopirox Olamine
The invention relates to a pharmaceutical composition for treating gynecologic inflammations. The pharmaceutical composition comprises ciclopirox olamine, palmatine and clindamycin or secnidazole serving as active pharmaceutical ingredients, wherein a synergistic effect is achieved among the three active pharmaceutical ingredients. The preferable preparation form of the pharmaceutical composition is gel. The product is uniform, fine and smooth in texture and appropriate in thickness. The pharmaceutical composition can be used for effectively treating mixed infectious diseases, particularly mixed infective vaginitis.
Owner:MUDANJIANG MEDICAL UNIV
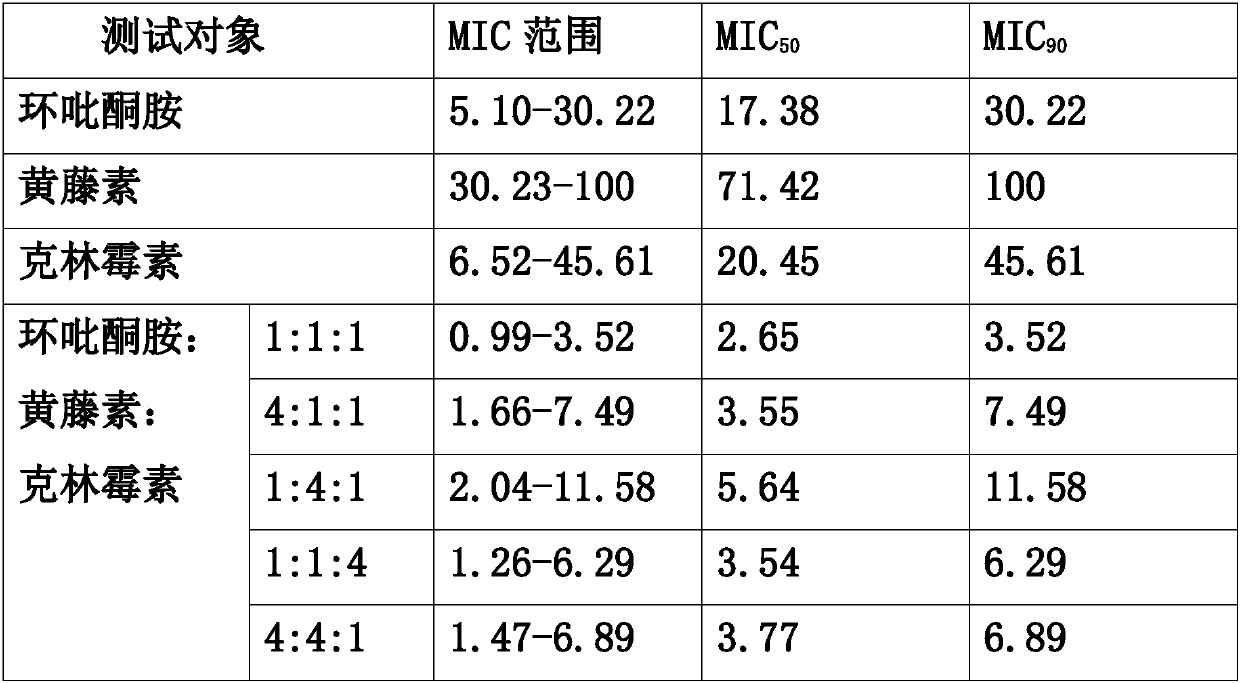
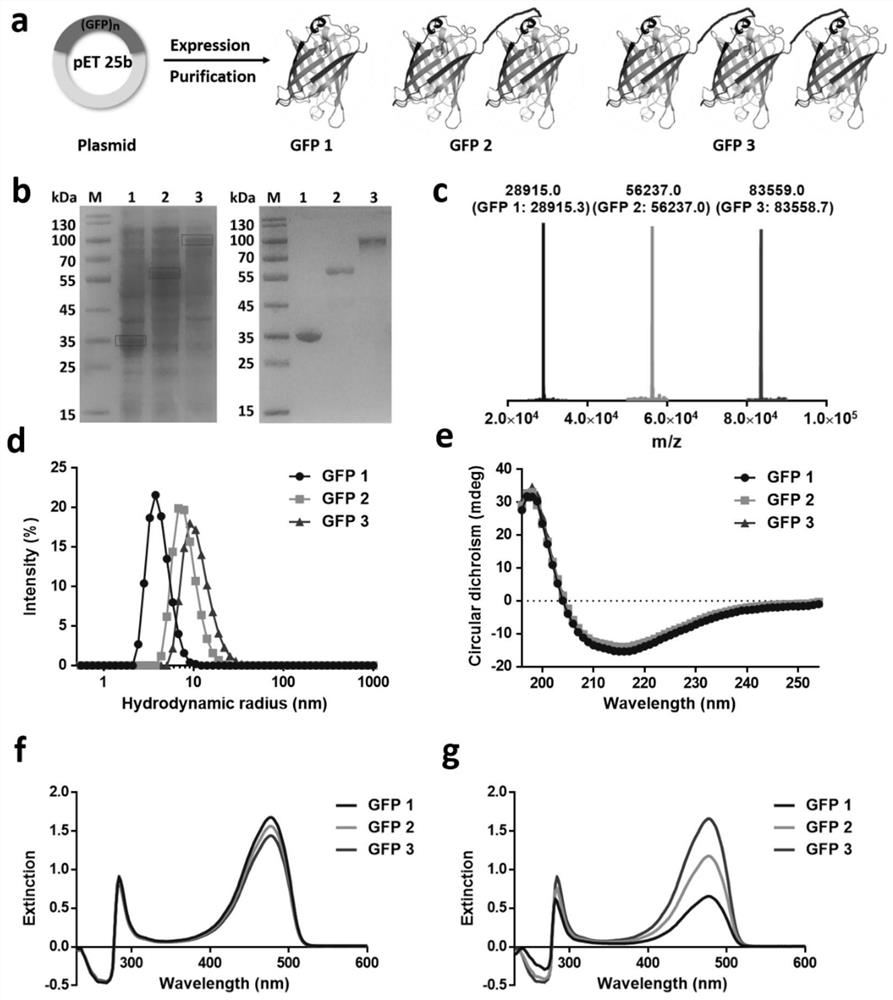
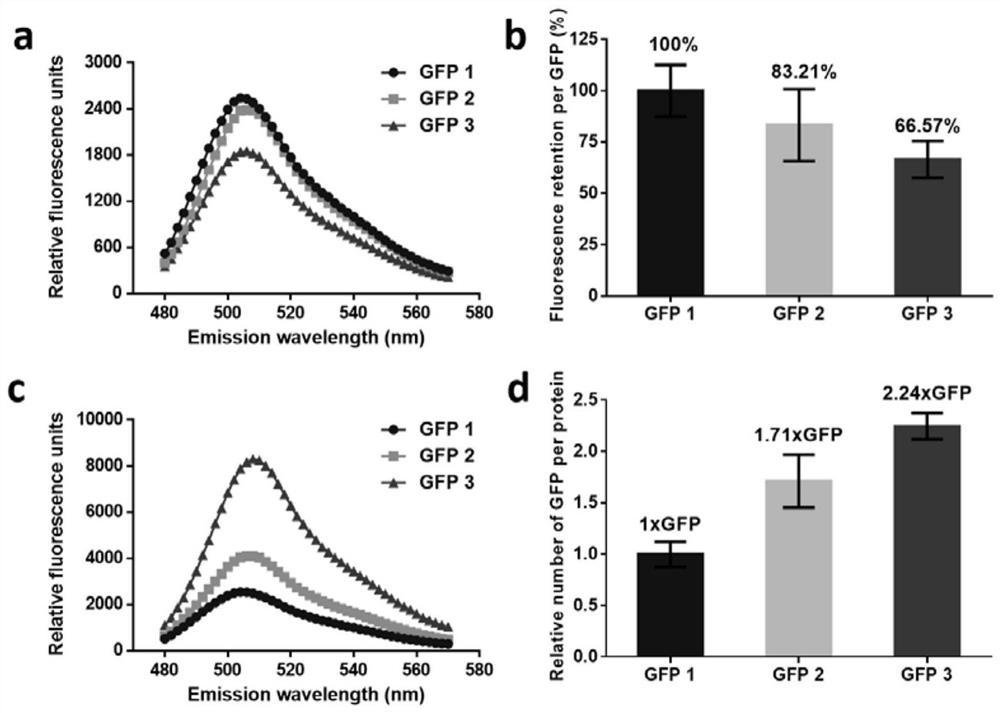
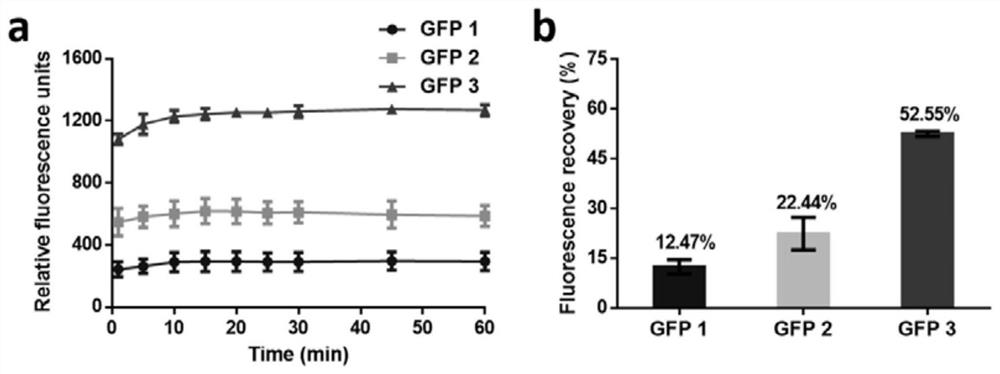
Self-fused concatenation protein modification method and application thereof
PendingCN113735980AImprove pharmaceutical propertiesImprove biological activityPeptide/protein ingredientsPharmaceutical non-active ingredientsPharmacometricsMouse tumor
The invention discloses a simple and universal protein modification method, in particular to a self-fused concatenation (SEC) protein modification method, which is used for improving the biological activity and the pharmaceutical property of protein. According to the invention, a group of GFP monomers, GFP dimers and GFP trimers are obtained through a genetic engineering technology. The GFP polymer can significantly improve the in-vitro biological activity and thermal stability of the GFP monomer and the residence time of mouse tumors, and the protein biological activity, thermal stability and tumor residence time are in positive correlation with the number of self-fused concatenation proteins. In addition, the IFN monomer, the IFN dimer and the IFN trimer are synthesized through the SEC technology, and the IFN polymer can remarkably improve the in-vitro biological activity, the in-vivo half-life period and the anti-tumor effect of the IFN monomer. The results show that the SEC can be used as a choice to replace the existing PEGylation or albumin fusion technology, and can be widely applied to other protein or small peptide drugs to improve the pharmacological characteristics, so that the long-acting protein or polypeptide drugs are successfully designed.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
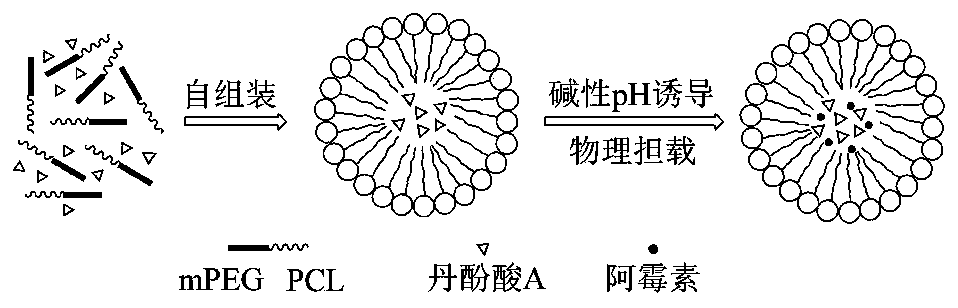
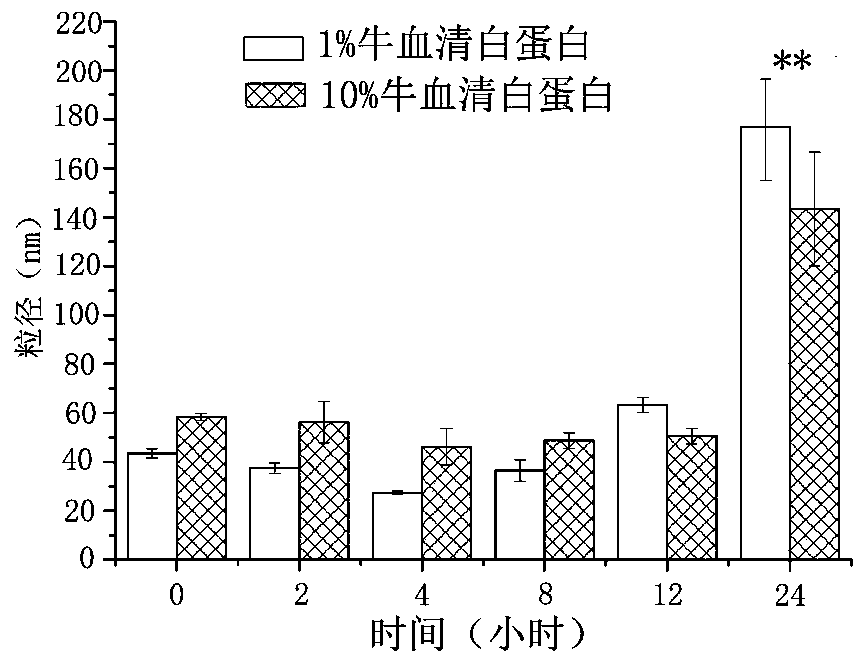
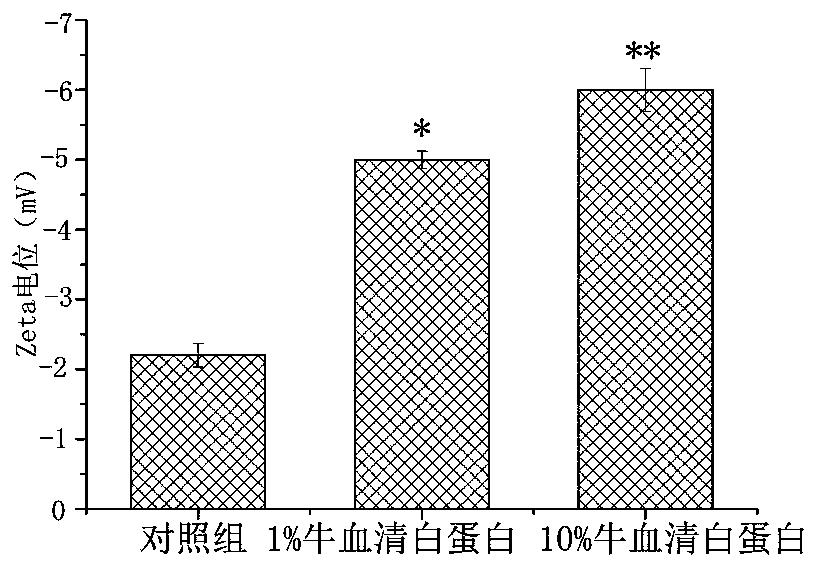
A kind of anthracycline antitumor antibiotic compound micelle and its preparation method and application
ActiveCN107693487BAchieving passive targetingAvoid damageOrganic active ingredientsPharmaceutical non-active ingredientsAntitumor AntibioticsPharmaceutical drug
The invention discloses compound nano micelles simultaneously loaded with anthracycline antitumor antibiotics and salvianolic acid A, and a preparation method and an application thereof. The compoundmicelles contain a therapeutically effective amount of anthracycline antitumor antibiotics, salvianolic acid A and an amphiphilic high-molecular polymer; through combined action of electrostatic attraction between an alkaline group of the anthracycline antitumor antibiotics and an acidic group of salvianolic acid A and self-assembly of the amphiphilic high-molecular polymer in an aqueous medium, the two drugs are jointly stably wrapped in nano micelles by a simple physical encapsulation mode; the obtained compound micelles have uniform particle size, have the encapsulation rate of 95% or more,and has both the drug loading amount and the stability obviously better than those of single-drug micelles.
Owner:YANTAI UNIV
The preparation method of rosiglitazone tartrate
ActiveCN102532122BImprove pharmaceutical propertiesLong lasting effectMetabolism disorderCarboxylic acid salt preparationDiabetic complicationAmino radical
The invention discloses a method for preparing rosiglitazone tartrate, relates to a method for preparing a novel medicinal compound, namely 5-[4-[2-methyl-N-(2-pyridyl) amino] ethoxy] thiazolidine-2,4-diketone tartrate, and belongs to the field of medicinal chemistry. The method comprises the following steps of: adding 5-[4-[2-methyl-N-(2-pyridyl) amino] ethoxy] thiazolidine-2,4-diketone into a polar solvent, adding tartaric acid with heating to dissolve the 5-[4-[2-methyl-N-(2-pyridyl) amino] ethoxy] thiazolidine-2,4-diketone, refluxing, cooling to room temperature after the reaction is finished, filtering, drying, and thus obtaining a product. The method is high in yield, short in reaction time, very simple in post treatment and convenient for industrialized production. The prepared compound can be used for treating type II diabetes and multiple diabetic complications, and is high in bioavailability and quick in response; and the pharmacokinetics of the compound is not affected by age.
Owner:KAIFENG PHARMA GRP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com