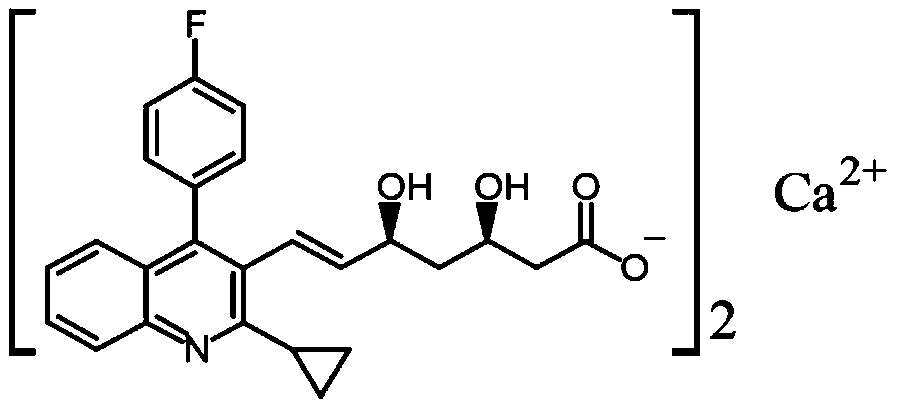
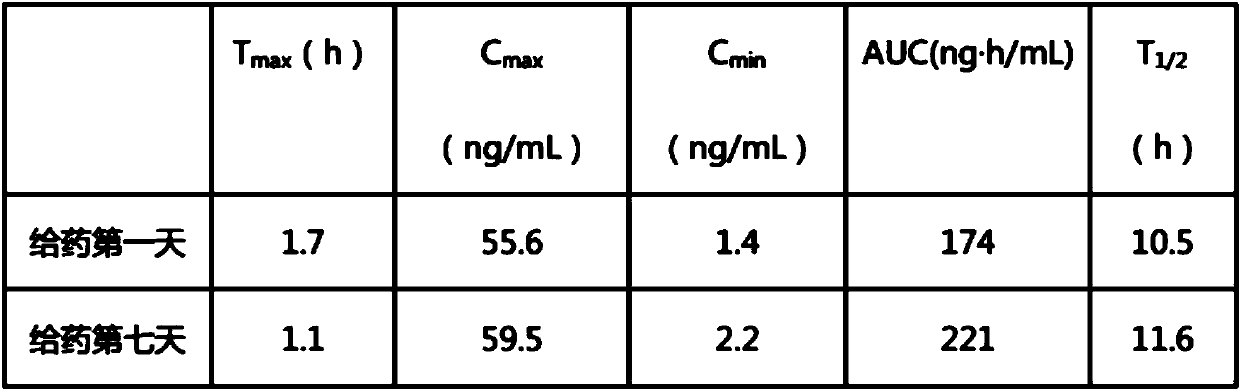
Pitavastatin calcium tablet pharmaceutical composition and its dry or wet preparation method
A technology of pitavastatin calcium and its composition, which is applied in the field of medicine, can solve the problems of insufficient use of active ingredients, easy configuration transformation, and lower cholesterol, and achieve easy control of the production process, high production efficiency, and batch-to-batch small difference effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Embodiment 1: preparation pitavastatin calcium tablet
[0184] Tablet prescription:
[0185] Pitavastatin Calcium 1mg,
[0186] Lactose 101.4mg,
[0187] Low-substituted hydroxypropyl cellulose 12mg,
[0188] Hydroxypropyl methylcellulose (2910) 2mg,
[0189] Magnesium aluminum silicate 2.4mg,
[0190] Magnesium stearate 1.2mg;
[0191] Chip method:
[0192] (1) Pitavastatin calcium is pre-mixed with a stabilizer and part lubricant (its amount is 8% of the amount of pitavastatin calcium) and ground into fine powder that can pass through 100 meshes to obtain pitavastatin calcium mixed powder; The materials are respectively crushed into fine powders that can pass through 80 meshes, and set aside;
[0193] (2) Add the filler to the wet mixing multifunctional granulator, and slowly add the pitavastatin calcium mixed powder obtained in step (1) and all or part of the disintegrant (adding 40%) in a stirring state, well mixed;
[0194] (3) Spray an additionally prep...
Embodiment 2
[0197] Embodiment 2: preparation pitavastatin calcium tablet
[0198] Tablet prescription:
[0199] Pitavastatin Calcium 1mg,
[0200] Lactose 120mg,
[0201] Low-substituted hydroxypropyl cellulose 4mg,
[0202] Hydroxypropylmethylcellulose (1828) 5mg,
[0203] Magnesium aluminum silicate 0.5mg,
[0204] Magnesium stearate 3mg;
[0205] Chip method:
[0206] (1) Pitavastatin calcium (using its pentahydrate, the feeding amount is converted into pitavastatin calcium) is pre-mixed and pulverized together with a stabilizer and part of the lubricant (the amount is 9% of the amount of pitavastatin calcium) into a fine powder that can pass through 100 meshes to obtain pitavastatin calcium mixed powder; the rest of the materials are respectively pulverized into fine powders that can pass through 80 meshes for subsequent use;
[0207] (2) Add the filler to the wet mixing multi-functional granulator, and slowly add the pitavastatin calcium mixed powder obtained in step (1) and...
Embodiment 3
[0211] Embodiment 3: preparation pitavastatin calcium tablet
[0212] Tablet prescription:
[0213] Pitavastatin Calcium 1mg,
[0214] Lactose (monohydrate) 40mg,
[0215] Low-substituted hydroxypropyl cellulose 20mg,
[0216] Hydroxypropyl methylcellulose (2208) 0.5mg,
[0217] Magnesium Aluminum Silicate 5mg,
[0218] Magnesium stearate 0.3mg;
[0219] Chip method:
[0220] (1) Pitavastatin calcium is pre-mixed together with stabilizer and part lubricant (its amount is 6% of the amount of pitavastatin calcium) and ground into fine powder that can pass through 100 meshes to obtain pitavastatin calcium mixed powder; The materials are respectively crushed into fine powders that can pass through 80 meshes, and set aside;
[0221] (2) Add the filler to the wet mixing multi-functional granulator, and slowly add the pitavastatin calcium mixed powder obtained in step (1) and all or part of the disintegrant (30%) in a stirring state, well mixed;
[0222] (3) Spray an addit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com