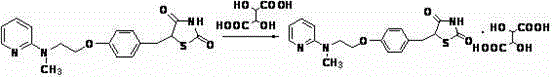The preparation method of rosiglitazone tartrate
A technology of tartrate and diketone, which is applied in the field of preparation of roger tartrate and new thiazolidinedione derivatives, can solve the problems of high price, limited clinical application, high price, etc., achieve short reaction time, good pharmaceutical properties, and fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0012] Example: Preparation of 5-[4-[2-methyl-N-(2-pyridyl)amino)ethoxy]thiazolidine-2,4-dione tartrate
[0013] Add 12g of 5-[4-[2-methyl-N-(2-pyridyl)amino)ethoxy]thiazolidine-2,4-dione into a 500mL reaction flask, then add 350mL of absolute ethanol and stir Until it is completely dissolved, heat to reflux until the system is clear, add 5.3g of tartaric acid, and continue to reflux and stir for 1h. After the reaction was completed, it was naturally cooled to room temperature, a white solid was precipitated, filtered, washed with absolute ethanol (10mL×3), and the product was air-dried at 60°C under normal pressure for 12h, with a yield of 95%. IR(KBr):3453,3342,3253,3070,2983,2929,2896,2783,1751,1697,1629,1540,1512,1461,1413,1351,1287,1234,1174,1133,1075,941,921,901,838,750,721,673,620,599,556,524,507cm -1 ; 1 HNMR(400MHz,DMSO): δ 12.3(s,2H),8.08(d, J =3.6Hz,1H),7.50(m,1H),7.13(d, J =8.4Hz,2H),6.87(d, J =8.4Hz,2H),6.64(d, J =8.4Hz,1H),6.56(t, J =6.0Hz,1H),5.13(m,1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com