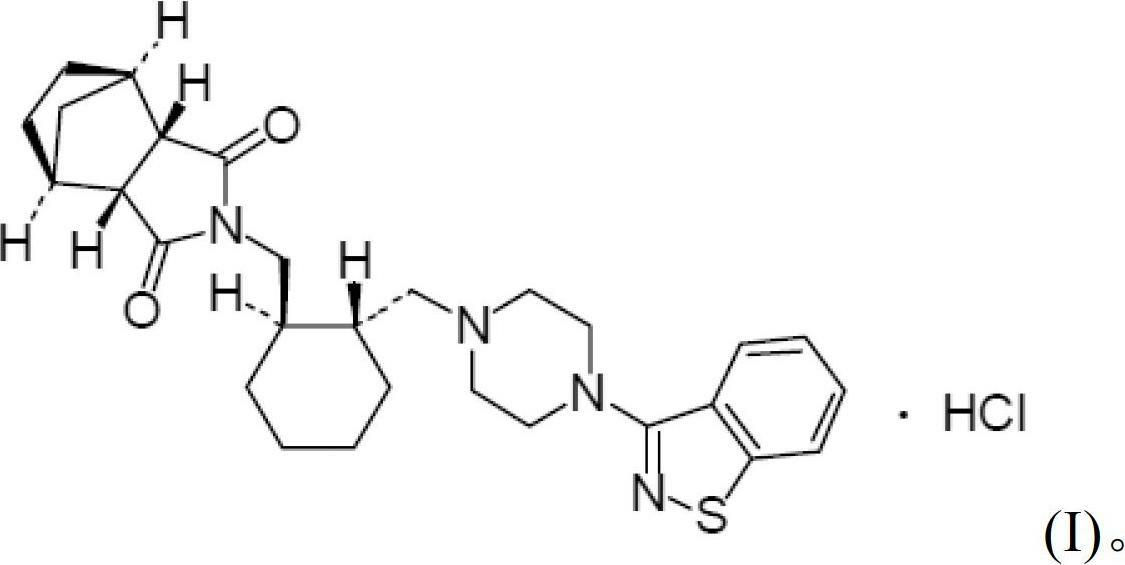
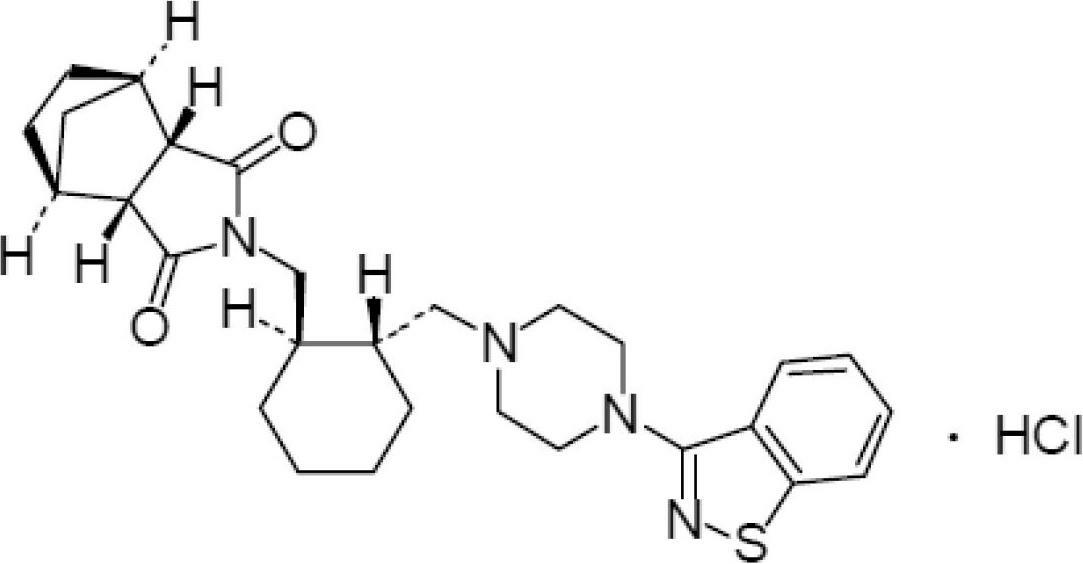
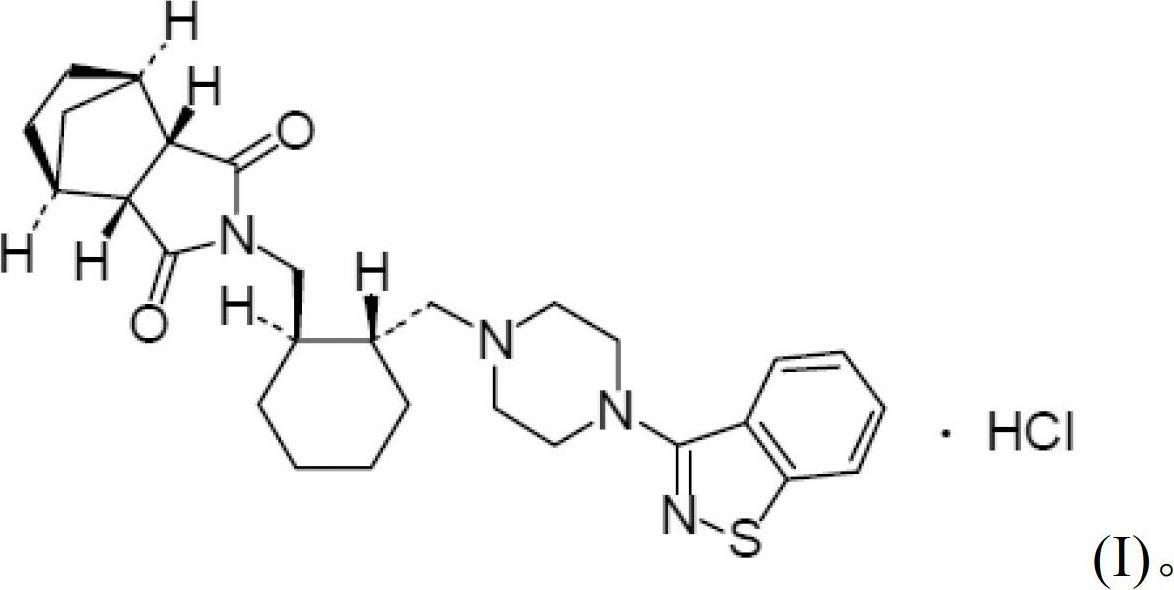
Lurasidone tablet and preparation method thereof
A technology of lurasidone tablet and lurasidone, which is applied in the field of lurasidone tablet and preparation, can solve the problem of not being able to obtain sufficient immediate release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0045] Embodiment 1-5: preparation tablet of the present invention
[0046] Formula (mg / tablet)
[0047] Component
[0048] Note: *HPMC is prepared as a 5% aqueous solution for use, and the final calculated amount is converted into the dry amount of the adhesive as shown in the formula of each example. For example, 5 in Example 2 uses the indicated adhesive, and the total amount added The amount is 5 mg of HPMC dry product. When there is a similar situation below, it also has a similar meaning. 10 mg of magnesium stearate shown in Example 4 is composed of 8 mg of magnesium stearate and 2 mg of colloidal silicon dioxide.
Embodiment 6-10
[0053] Examples 6-10: Preparation of tablets of the present invention
[0054] Formula (mg / tablet)
[0055] Component
[0056] *PVP K30 is used as a 5% aqueous solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com