Application of Icaritin in preparation of drugs for curing GP130 related diseases
A technology of GP-130 and Acradine, which is applied in the field of medicine to achieve good pharmaceutical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of Alcoradine
[0033] Acradine, also known as icariin, is extracted and separated from the traditional Chinese medicine Epimedium.
[0034] The preparation method of Epimedium is disclosed in the patent whose publication number is CN 101302548. In the method, icariin is used as a raw material, hydrolyzed by β-glucosidase, the precipitate obtained by centrifuging the hydrolyzed product is dissolved in acetone, and the supernatant is obtained by centrifuging and filtering. Then the supernatant liquid obtained by centrifugation is recrystallized with water to obtain the pure Epimedium. The icariin in the present invention was purchased from Shaanxi Jiahe Plant Chemical Co., Ltd., with a purity of 90%.
Embodiment 2
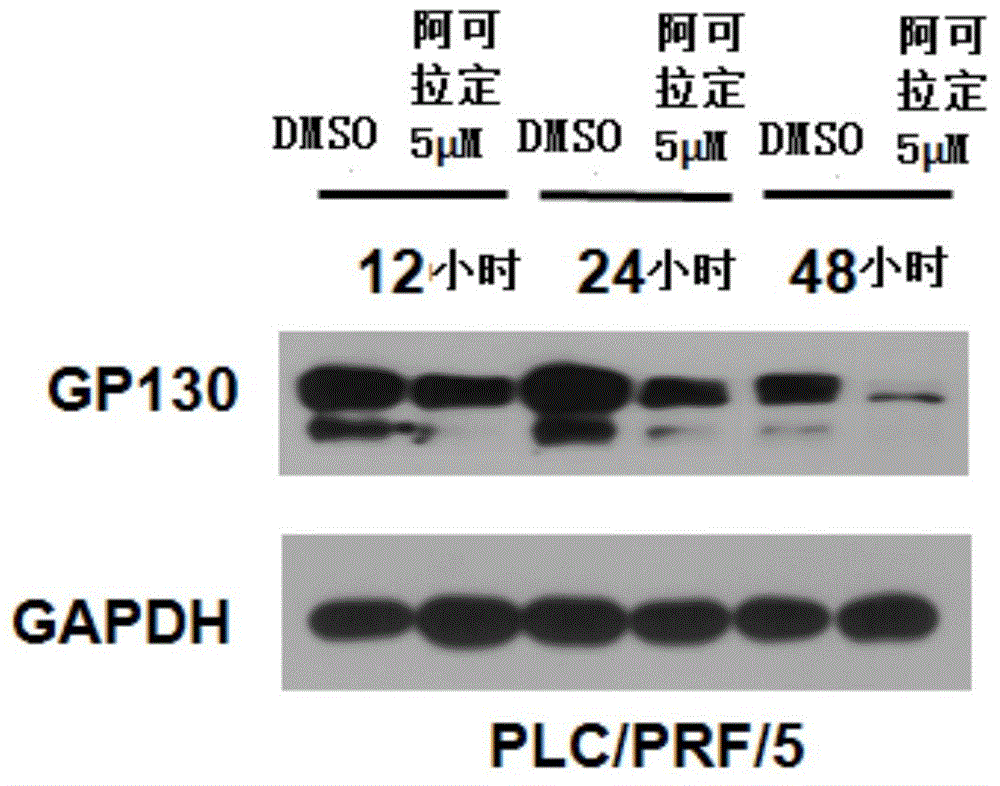
[0036] Detect the inhibitory effects of alcoladine on hepatocarcinoma PLC / PRF / 5 cells.
[0037] Experimental steps: cell culture: first, the PLC / PRF / 5 liver cancer cells are cultured to a cell density of 80% in an amino acid and glucose medium (i.e., DMEM medium) containing a volume concentration of 10% fetal bovine serum; The cells were divided into phenol red-free medium containing activated carbon-treated fetal bovine serum at a volume concentration of 2.5%, and cultured for 24 hours.
[0038] Add dimethyl sulfoxide (DMSO) and alcradine: add DMSO control (that is, the DMSO solution not containing alcradine) and 5 μmol / L alcradine DMSO solution respectively in the culture system, and make each The final volume concentration of DMSO in the reaction system was 0.1%.
[0039] The cells were harvested at 12, 24 and 48 hours, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and GP130 antibodies were added to the cells for detection by western blot.
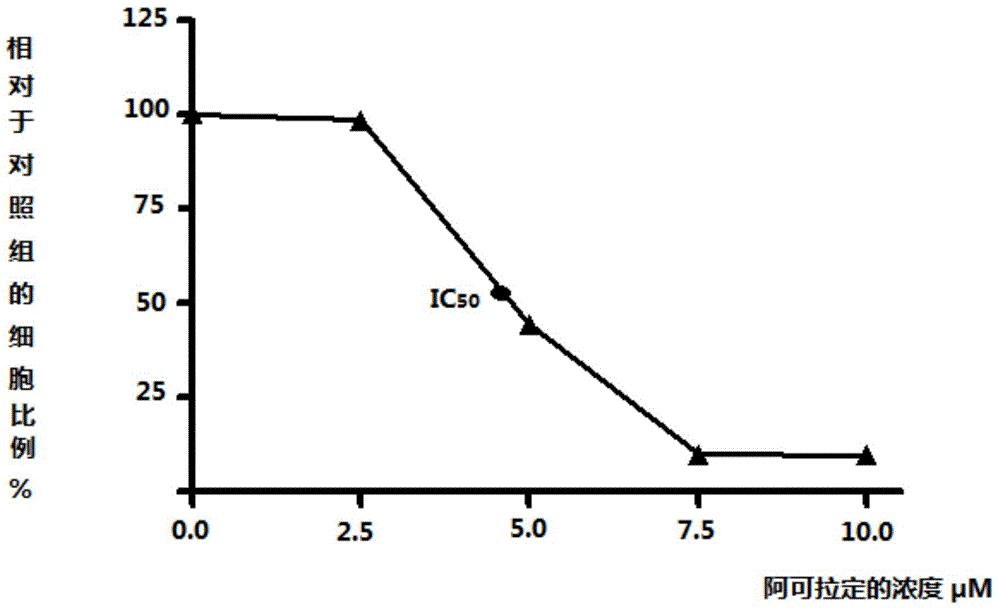
[0040] Test result pass...
Embodiment 3
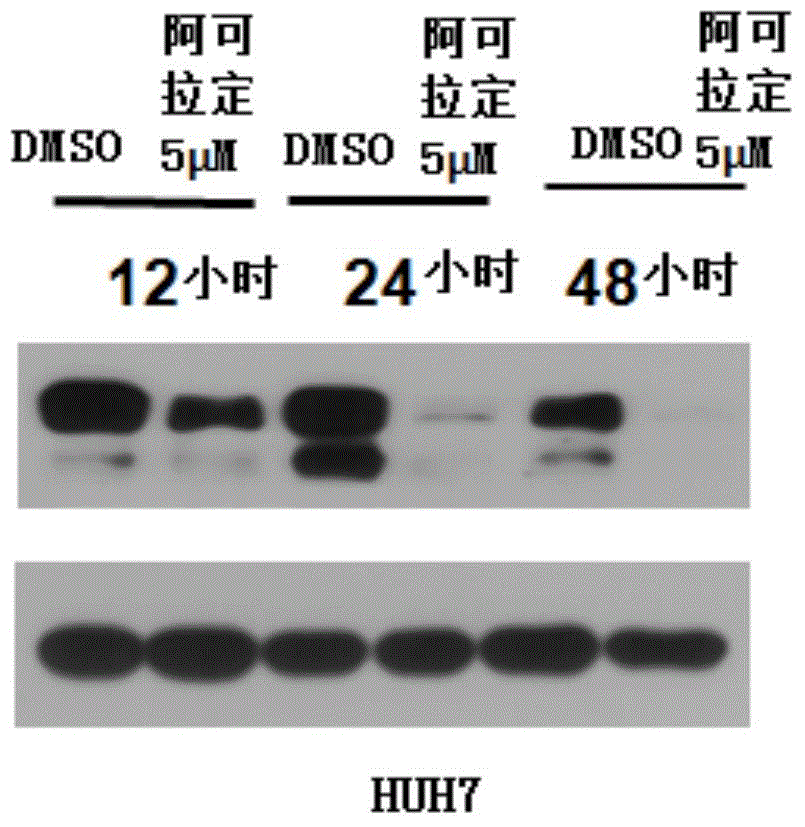
[0042] To detect the inhibitory effect of alcoladine on the liver cancer cell HUH7.
[0043] Experimental steps: cell culture: firstly culture HUH7 cells in amino acid and glucose medium (ie DMEM medium) containing 10% volume concentration of fetal bovine serum to a cell density of 80%; In the phenol red-free medium with a volume concentration of 2.5% fetal bovine serum, culture for 24 hours.
[0044] Add dimethyl sulfoxide (DMSO) and alcradine: add DMSO control (that is, the DMSO solution that does not contain alcradine) and the alcladine DMSO solution of 5 μ M concentration respectively in the culture system, and make each The final volume concentration of DMSO in the reaction system was 0.1%.
[0045] The cells were harvested at 12, 24 and 48 hours, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and GP130 antibodies were added to the cells for detection by western blot.
[0046] Test result passed figure 2 It can be seen in the upper half of the figure that the ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com