Compound and crystals thereof
A technology of compounds and crystals, applied in the field of pharmacy, can solve the problems of low biological activity, inconsistent with drug characteristics, and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
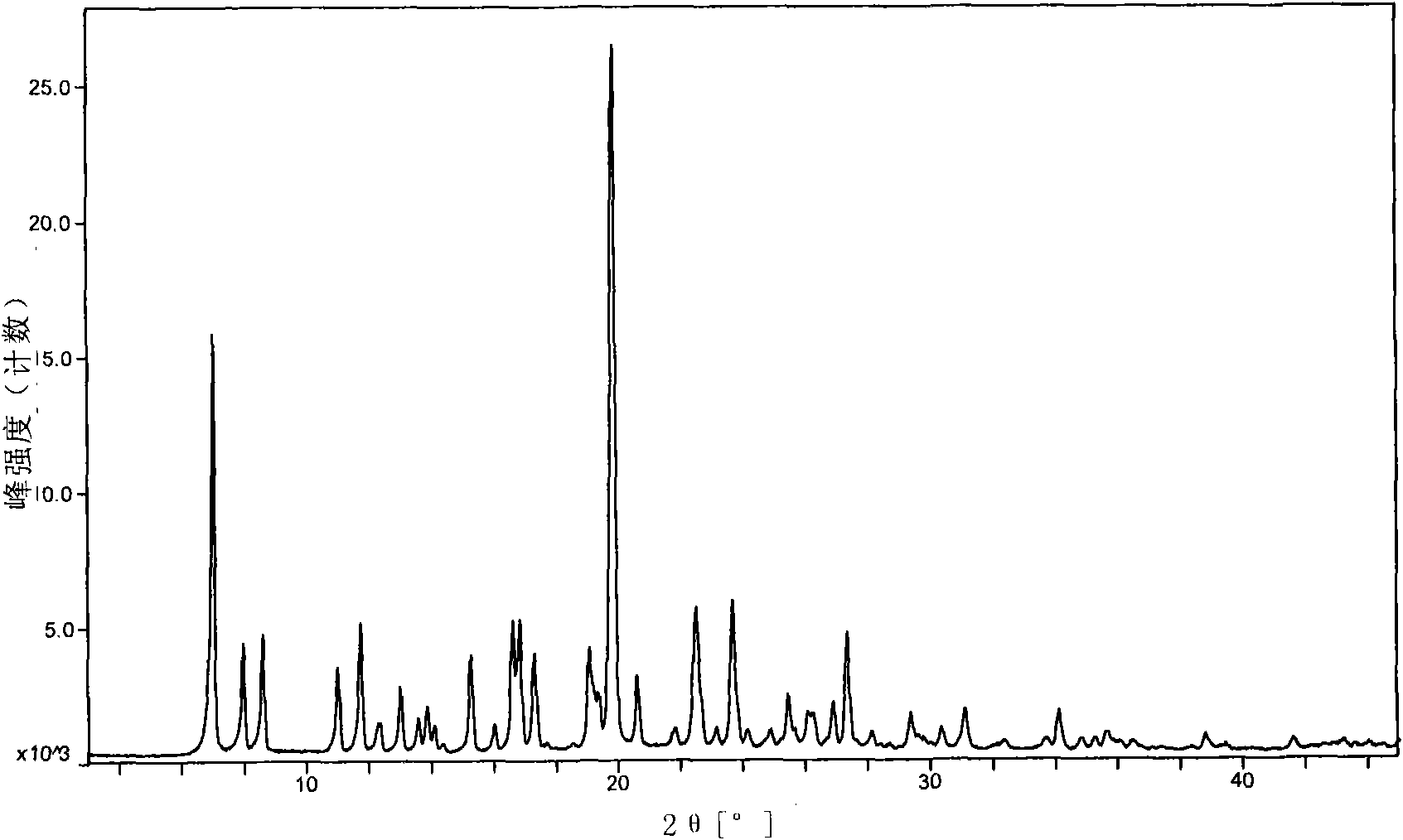
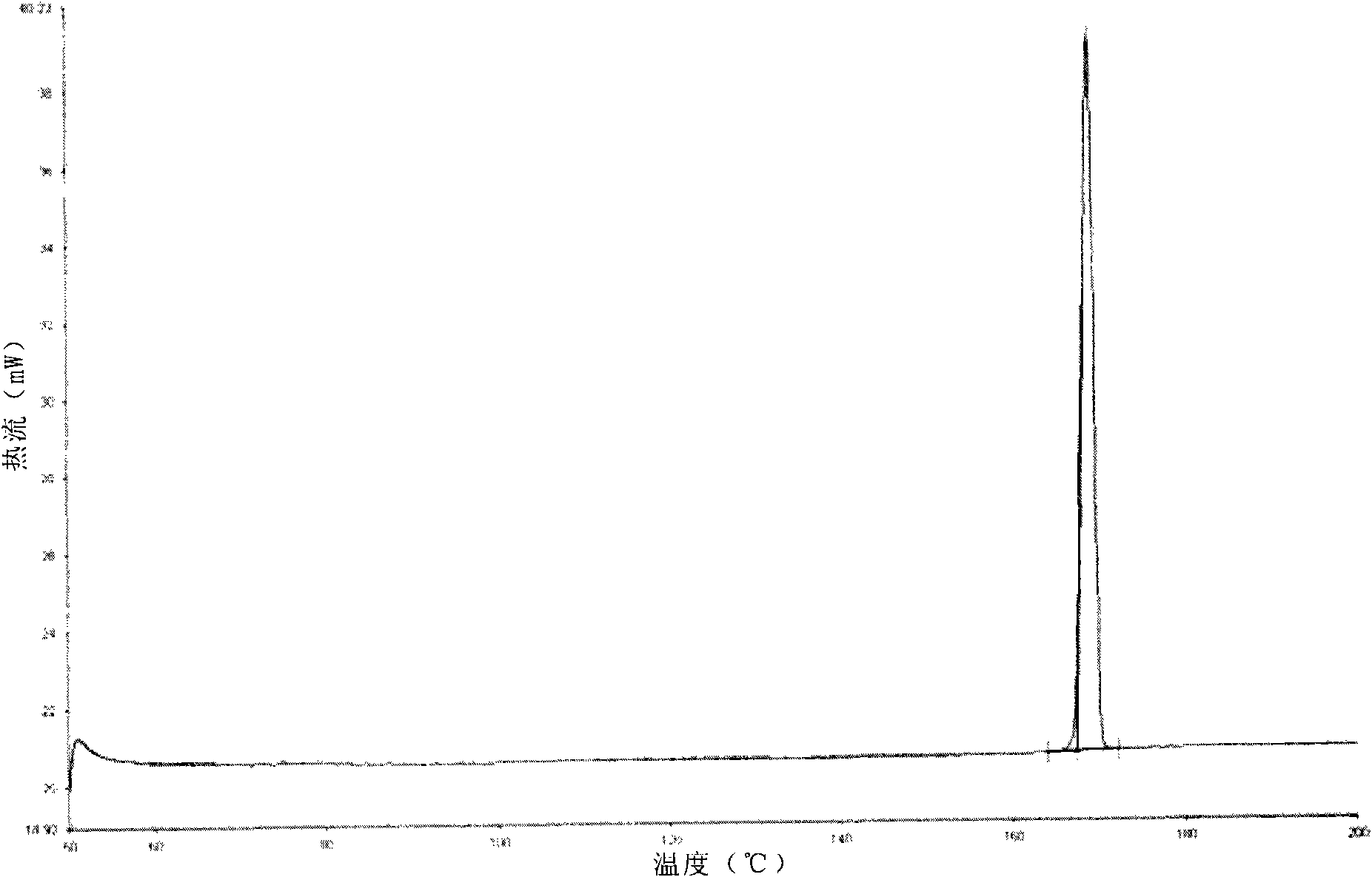
Image
Examples
preparation example Construction
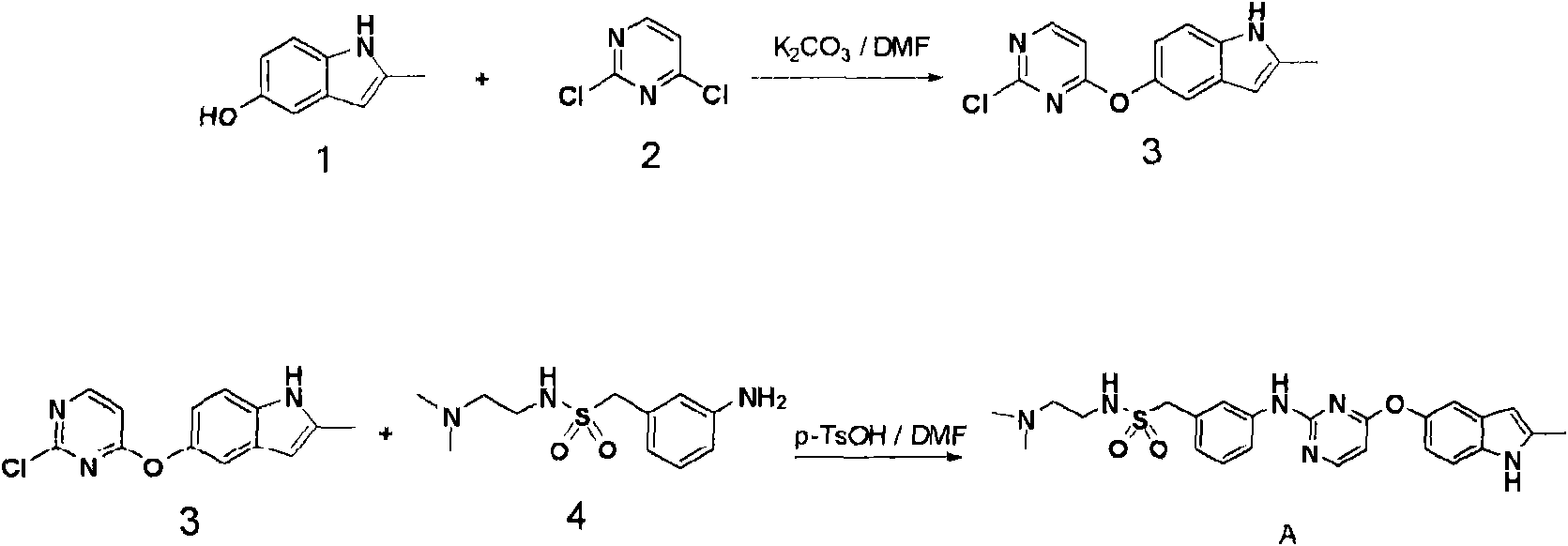
[0062] The compound can be prepared by the attached figure 1 route is realized.
[0063] The first step: the synthesis of 5-(2-chloropyrim-5-oxo)-2-methyl-1H-indole (compound 3)
[0064] Under low temperature and alkaline conditions, in an appropriate solvent, the hydroxyl group of 5-hydroxyl-2-methylindole (compound 1) selectively attacks the 4-position of 2,4-dichloropyrimidine (compound 2), Form intermediate 5-(2-chloropyrim-5-oxo)-2-methyl-1H-indole (compound 3); wherein, the base used can be an inorganic base, such as NaHCO 3 , KOH, NaOH, K 2 CO 3 , KHCO 3 , or organic bases, such as diisopropylethylamine, pyridine, triethylamine, trimethylamine, etc.; the reaction solvent can be acetonitrile, N, N-dimethylformamide, dioxane, tetrahydrofuran, etc.; the reaction temperature It is suitable to be controlled within the range of 0~60 degrees;
[0065] The second step: N-[2-(dimethylamino)ethyl]-[3-[4-(2-methyl-1H-indole-5-oxyl)pyrimidine-2-amino]phenyl]methanol Synthesi...
Embodiment 1
[0168] Synthesis of N-[2-(dimethylamino)ethyl]-[3-[4-(2-methyl-1H-indole-5-oxyl)pyrimidine-2-amino]phenyl]methanesulfonamide
[0169] Preparation of compound 5-(2-chloropyrim-5-oxo)-2-methyl-1H-indole (compound 3)
[0170] Add 3kg of 5-hydroxy-2-methylindole (compound 1), 9L of acetonitrile, and 4.2Kg of potassium carbonate into a 50L reaction flask, stir and cool down to 0-5°C under nitrogen protection; slowly add 2,4-dichloro Pyrimidine (compound 2) 3.05kg of acetonitrile solution; after dripping, the temperature was raised to 5-10°C, and the reaction was stirred for 4-8 hours; under stirring, the reaction solution was transferred to a 100L treatment tank with 54L of pure water, and the solid was precipitated. Stir at room temperature for 1 hour. Filter, wash the filter cake with pure water, and dry to obtain 5.1 kg of the product, with a purity of 98.6% and a yield of 96%.
[0171] Synthesis of N-[2-(dimethylamino)ethyl]-[3-[4-(2-methyl-1H-indole-5-oxyl)pyrimidine-2-amin...
Embodiment 2
[0175] Determination of the inhibitory effect of compound A on KDR activity using Z’-lyte detection kit
[0176] Experimental materials and experimental methods: using Z′-LYTE TM Tyr1 substrate peptide kit (Invitrogen, Cat. PV3190) was used to detect the inhibitory effect of compound A on KDR kinase activity in vitro. The detection reaction contained 300 ng / mL recombinant human KDR kinase catalytic region (Invitrogen, Cat. PV3660), 10 μM ATP, 1 μM fluorescent double-labeled substrate peptide and different concentrations of compound A obtained in Example 1. Reactions were performed in black 384-well plates (Thermolabsystems, Cat. 7805). According to the method recommended by the manufacturer, the enzyme catalyzes the reaction at room temperature for 1 hour, then adds a fluorescence accelerator, continues to react at room temperature for 1 hour, and then adds a terminator to terminate the reaction. The inhibition rate of the compound on the enzyme reaction was calculated acco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com