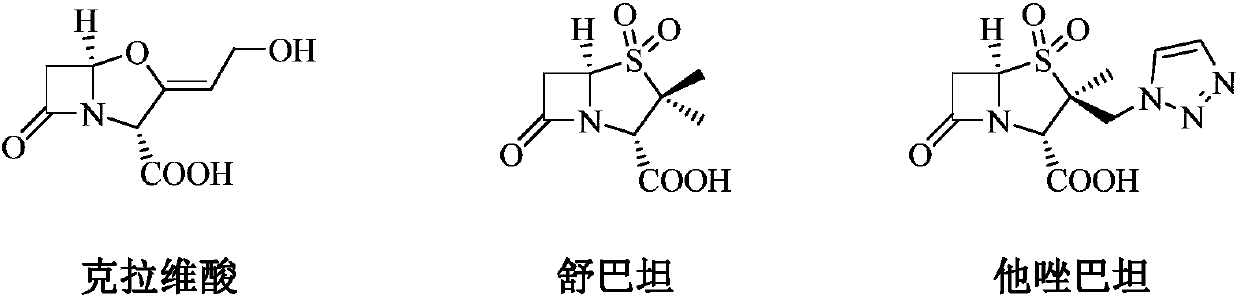
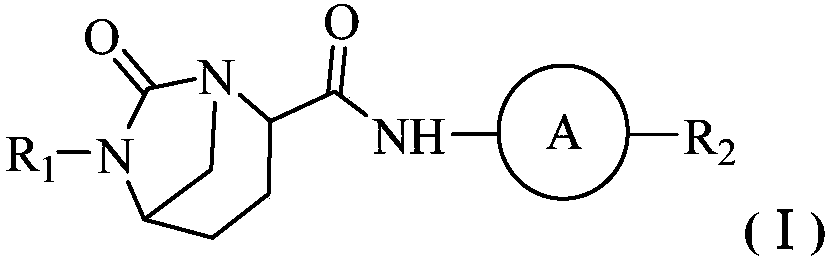
Antibacterial combination and application thereof
A compound and solvent compound technology, applied in the field of medicine, can solve the problems of limited application, not showing good drug effect, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0199] The in vitro antibacterial activity of experimental example 1 compound (a)
[0200] Bacteria to be tested: standard enzyme-producing strains used in experiments were purchased from ATCC, and clinically isolated CRE strains were purchased from Southwest Hospital of Third Military Medical University.
[0201] Test product: part of compound (a) or the salt of compound (a), its chemical name and preparation method are shown in the preparation examples of each compound.
[0202] Control drugs: avibactam (AVI) sodium salt and MK-7655 were all made by Shandong Xuanzhu Pharmaceutical Technology Co., Ltd., and their structural formulas are as described in the background technology.
[0203] Experimental method: agar dilution method, refer to M100-S23: Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement (Clinical And Laboratory Standards Institute, 2013), calculate the minimum inhibitory concentration (MIC, minimum inhibitory conc...
experiment example 2
[0216] In vitro enzymatic activity test of experimental example 2 compound (a)
[0217] Test product: part of compound (a) or the salt of compound (a), its chemical name and preparation method are shown in the preparation examples of each compound.
[0218]Control drugs: avibactam (AVI) sodium salt and MK-7655 were all made by Shandong Xuanzhu Pharmaceutical Technology Co., Ltd., and their structural formulas are as described in the background technology.
[0219] experimental method:
[0220] Nitrocefin (Nitrocefin, cephalosporin antibiotics) is sensitive to most β-lactamases and will change color after being hydrolyzed. The hydrolysis rate of Nitrocefin was determined by recording the corresponding absorbance in the reaction system in real time. β-lactamase inhibitors will inhibit the enzyme's hydrolysis of Nitrocefin and reduce the rate of hydrolysis. Calculate the IC of the inhibitor by measuring the reaction rate in the same reaction system under different inhibitor co...
experiment example 3
[0240] The in vitro antibacterial activity of experimental example 3 compound (a) combined with ceftazidime
[0241] Test article: part of compound (a) or the salt of compound (a), its chemical name and preparation method are as above.
[0242] Control drug: Avibactam (Avibactam, AVI) sodium salt, purchased from Jinan Xinzheng Pharmaceutical Technology Co., Ltd.; MK-7655, self-made, referring to the preparation method in WO2009091856A2 (public date 2009-07-23); ceftazidime (ceftazidime , CAZ), purchased from Nanjing Shenglide Biotechnology Co., Ltd.
[0243] Bacteria to be tested: standard enzyme-producing strains for experiments were purchased from ATCC, and clinically isolated CRE strains were purchased from Third Military Medical University
[0244] Learn Southwest Hospital.
[0245] Experimental method: agar dilution method, refer to M100-S23: Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement (Clinical And Laboratory St...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com