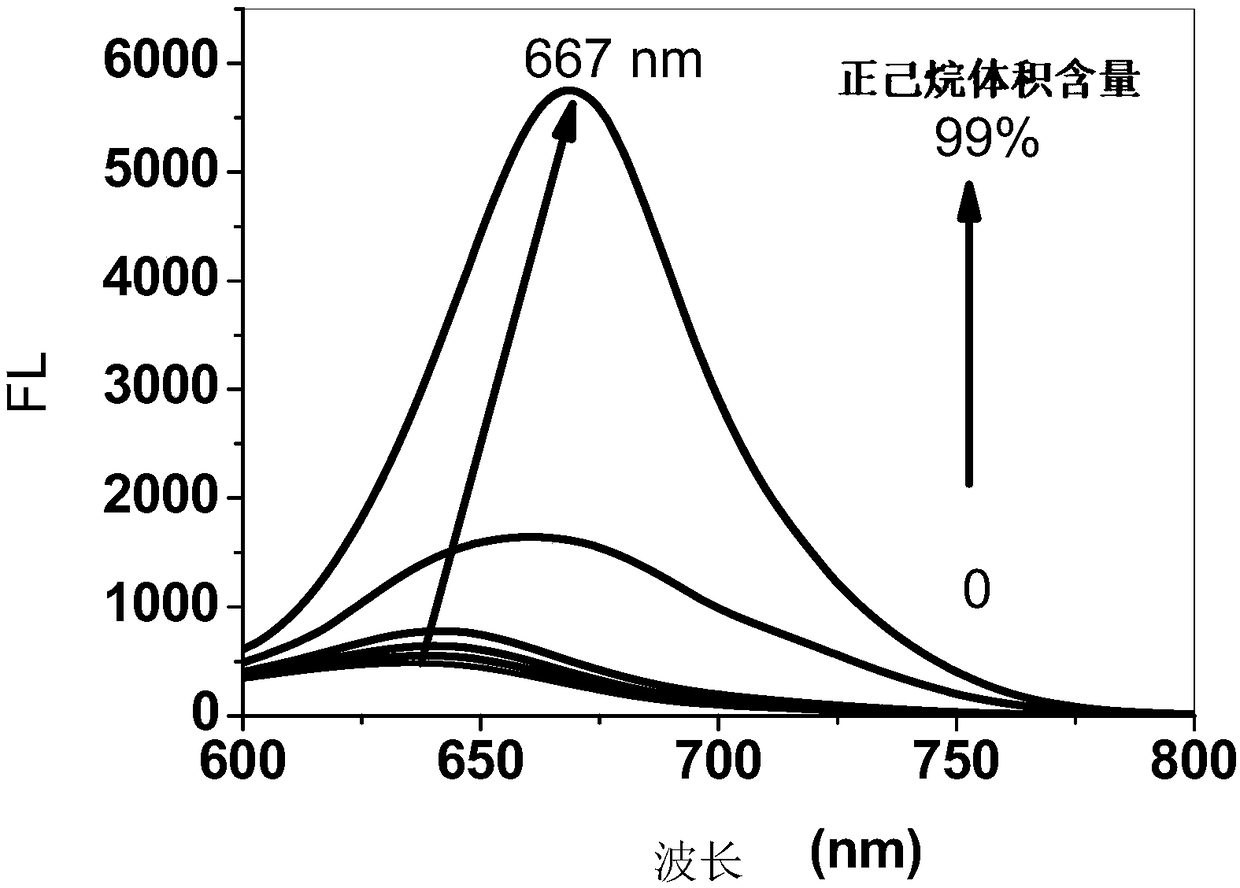
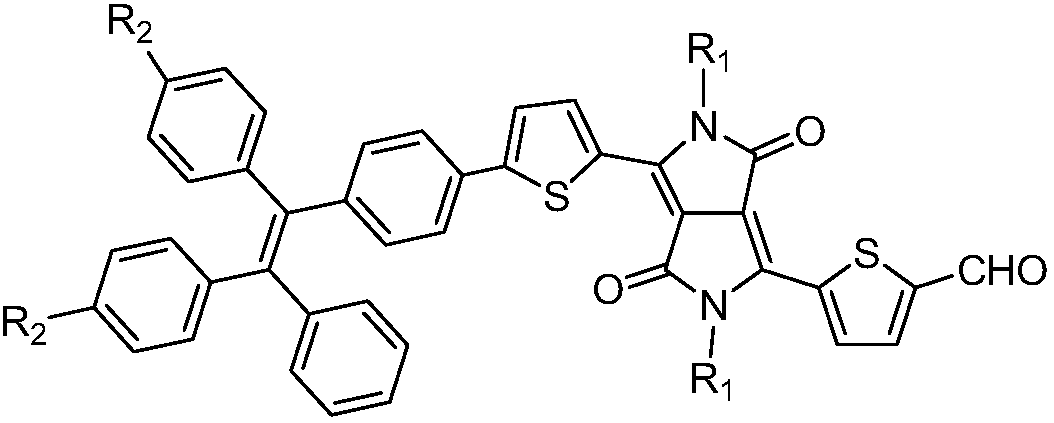
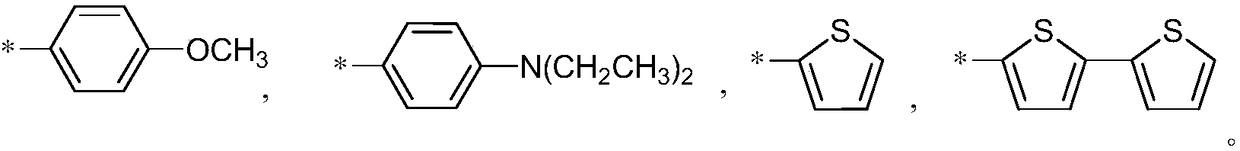Aggregation-induced emission near-infrared emission diketopyrrolopyrrole compound and preparation method thereof
A diketopyrrolopyrrole, aggregation-induced luminescence technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve problems such as scarcity of species, and achieve the effect of convenient derivatization and functionalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of aggregation-induced luminescent near-infrared emitting diketopyrrolopyrrole compound DPP1
[0034] (1) 3,6-dithienylpyrrolopyrrole diketone was prepared according to the method disclosed in literature Li F C, Qiao H B, Guo K, Zhou L, Chen X G, RSC Adv., 2014, 4, 58027-58035, 2 -(4-((Z)-1,2-bis(diethylamino)-2-styrene)phenyl-4,4,5,5-tetramethyl)boronic acid pinacol ester According to literature Shen X Y , prepared by the method disclosed in Wang Y J, ZhaoE, The J.of Phys.Chem.C, 2013, 117(14):7334-7347.
[0035] (2) In 20mmol of potassium tert-butoxide and 100mL of dry N-methylpyrrolidone solution, add 10mmol of 3,6-dithienylpyrrolopyrrole diketone and 10mmol of bromoisoctane, under argon protection Heat to reflux for 16h, cool to room temperature, filter with suction, and distill the filtrate under reduced pressure to remove most of N-methylpyrrolidone, then wash with water, CH 2 Cl 2 Extraction, anhydrous NaSO 4 Drying and column chromatog...
Embodiment 2
[0040] Example 2: Preparation of aggregation-induced luminescent near-infrared emitting diketopyrrolopyrrole compound DPP2
[0041] (1) 3,6-dithienylpyrrolopyrrole diketone was prepared according to the method disclosed in literature Li F C, Qiao H B, Guo K, Zhou L, Chen X G, RSC Adv., 2014, 4, 58027-58035, 2 -(4-((Z)-1,2-bis(dimethoxy)-2-styrene)phenyl-4,4,5,5-tetramethyl)boronic acid pinacol ester according to literature Hu F , prepared by the methods disclosed in Huang Y, Zhang G, Zhao R, Zhang D Q, Tetrahedron Lett.2014, 55, 1471-1474.
[0042] (2) In 20mmol of potassium tert-butoxide and 100mL of dry N-methylpyrrolidone solution, add 10mmol of 3,6-dithienylpyrrolopyrrole diketone and 10mmol of bromoisoctane, under argon protection Heat to reflux for 16h, cool to room temperature, filter with suction, and distill the filtrate under reduced pressure to remove most of N-methylpyrrolidone, then wash with water, CH 2 Cl 2 Extraction, anhydrous NaSO 4 Drying and column chro...
Embodiment 3
[0046] Example 3 Preparation of aggregation-induced luminescent near-infrared emitting diketopyrrolopyrrole compound DPP3
[0047] (1) 1) 3,6-dithienylpyrrolopyrrole diketone was prepared according to the method disclosed in literature Li F C, Qiao H B, Guo K, Zhou L, Chen X G, RSC Adv., 2014, 4, 58027-58035 , 2-(4-((Z)-1,2-bis(diethylamino)-2-styrene)phenyl-4,4,5,5-tetramethyl)boronic acid pinacol ester according to literature Prepared by the method disclosed in Shen X Y, Wang Y J, ZhaoE, The Journal of Physical Chemistry C, 2013, 117(14): 7334-7347.
[0048] (2) In 20mmol of potassium tert-butoxide and 100mL of dry N-methylpyrrolidone solution, add 10mmol of 3,6-dithienyldiketopyrrolopyrrole and 10mmol of bromododecane, under argon protection Heated to reflux for 5h, cooled to room temperature, suction filtered, and the filtrate was distilled off under reduced pressure to remove most of N-methylpyrrolidone, washed with water, CH 2 Cl 2 Extraction, anhydrous NaSO 4 Drying...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com