Synthetic method of 2-chlorine-5-fluorine-6-ethylpyrimidin-2-amine
A technique for the synthesis of ethylpyrimidine and its synthesis method, which is applied in the synthesis of 2-chloro-5-fluoro-6-ethylpyrimidine and the synthesis of pyrimidine derivatives, and can solve the problems of long synthetic routes, environmental pollution, and low yields , to avoid pollution, improve safety and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
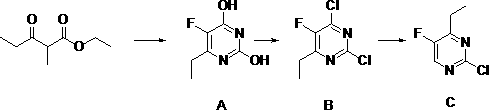
[0023] Synthesis of Compound A:
[0024] In a 3-liter three-necked flask, add 180 grams of sodium methoxide and 1500 milliliters of methanol, fully dissolve, cool down to room temperature, add 100 grams of urea, stir for half an hour, dropwise add 220 grams of ethyl 2-fluoropropionyl acetate, add After heating to reflux for 2 hours, the solvent was evaporated, dissolved in water, acidified with dilute hydrochloric acid, adjusted to pH 2.1, filtered and washed with water to obtain 150 g of Intermediate A, with a purity greater than 95% and a yield of 69.92%.
[0025] Synthesis of Compound B:
[0026] Suspend 150 grams of intermediate A in 700 grams of phosphorus oxychloride, add 200 grams of triethylamine dropwise at 39 ° C, stir at room temperature for half an hour, then heat up to 100 ° C for chlorination reaction for 2 hours, the mixture After cooling, it was concentrated under reduced pressure to remove excess phosphorus oxychloride, quenched by adding water, and then extr...
Embodiment 2
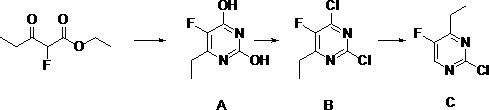
[0032] In a 3-liter three-necked flask, add 216 grams of sodium methoxide and 1800 milliliters of methanol, fully dissolve, cool down to room temperature, add 120 grams of urea, stir for half an hour, dropwise add 264 grams of ethyl 2-fluoropropionyl acetate, add After warming up to the reflux reaction for 2.5 hours, the solvent was evaporated, dissolved in water, acidified with dilute hydrochloric acid, adjusted to pH 2.3, filtered, washed with water to obtain 180 g of Intermediate A, the purity was greater than 95%, and the yield was 66.42%.
[0033] Synthesis of Compound B:
[0034] Suspend 180 grams of intermediate A in 1100 grams of phosphorus oxychloride, add 120 grams of diisopropylethylamine dropwise at 30 ° C, stir at room temperature for half an hour, and then heat up to 100 ° C for chlorination reaction 5 hours, the mixture was cooled, concentrated under reduced pressure to remove excess phosphorus oxychloride, quenched with water and then extracted with ethyl aceta...
Embodiment 3
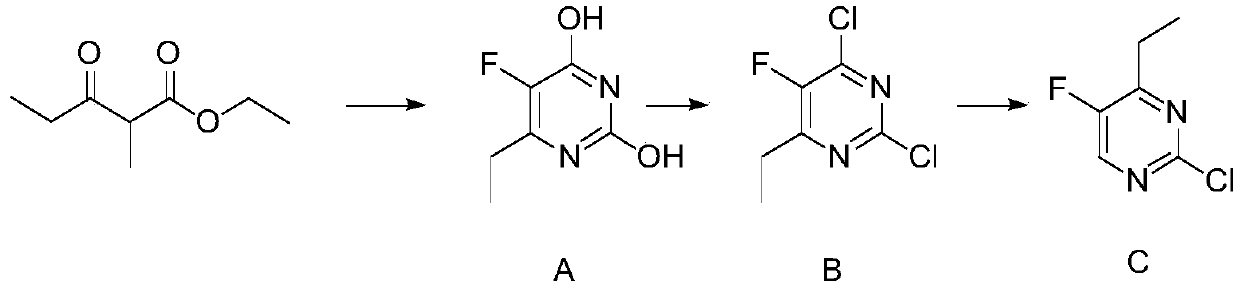
[0038] In a 3-liter three-necked flask, add 270 grams of sodium methoxide and 2250 milliliters of methanol, fully dissolve, cool down to room temperature, add 150 grams of urea, stir for half an hour, dropwise add 330 grams of ethyl 2-fluoropropionyl acetate, add After warming up to reflux for 3 hours, the solvent was evaporated, dissolved in water, acidified with dilute hydrochloric acid, adjusted to pH 2.9, filtered, washed with water to obtain 232 g of Intermediate A, with a purity greater than 95% and a yield of 68.48%
[0039] Synthesis of Compound B:
[0040] Suspend 230 grams of intermediate A in 2050 grams of phosphorus oxychloride, add 250 grams of triisopropylamine dropwise at 35 ° C, stir at room temperature for half an hour, and then heat up to 100 ° C and carry out chlorination reaction for 4.5 hours, The mixture was cooled and concentrated under reduced pressure to remove excess phosphorus oxychloride, quenched by adding water, and then extracted with ethyl aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com